Introduction
The connection between malignancy and thrombosis has long been established.1 Despite the increased risk of venous and arterial thrombosis, stroke as the first clinical manifestation of malignancy is rare.2,3Hypercoagulability is the most common underlying mechanism, followed by nonbacterial thrombotic endocarditis and chronic Disseminated intravascular coagulation (DIC).2,3The latter patients have less conventional stroke risk factors, higher D-dimer levels and multifocal lesions more frequently.3We describe the case of a patient with multisystemic microthrombosis in the setting of subclinical DIC and underlying occult lung adenocarcinoma, presenting as an ischemic stroke.
Case Report
An 80-year-old male presented to the Emergency Department with dysarthria and right central facial palsy with several hours of evolution. On physical examination, he had dysarthria and right central facial palsy with no other neurological signs. The skin was pale, there were purpuric lesions on his legs and a systolic murmur on cardiac auscultation was noted. There was no peripheral edema. He was a former smoker and his medical history included hypertension, diabetes, dyslipidemia, stage two chronic kidney disease and chronic disease anemia. He had no previous history of thrombotic or hemorrhagic events. He was medicated for his comorbidities and no new drugs were recently introduced.
Brain magnetic resonance imaging showed a left fronto-insular cortico-subcortical and a right cerebellar lesion, both with hyperintense signal in T2 and FLAIR and a restric-tion pattern in the diffusion, compatible with recent ischemic etiology (Fig. 1). He was started on aspirin 100 mg qd and enoxaparin 40 mg qd.
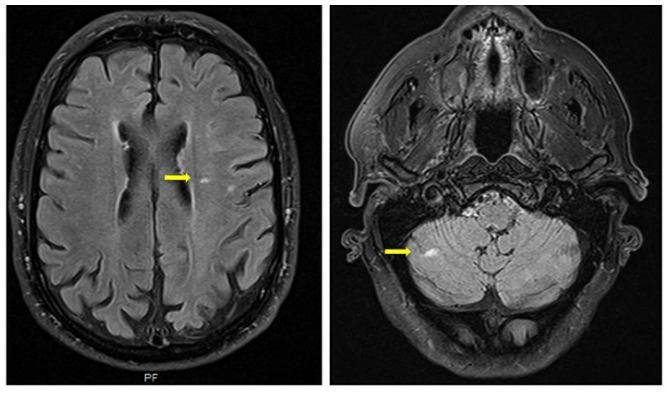
Figure 1: Multiple bilateral ischemic lesions seen on T2-weighted images of brain magnetic resonance (yellow arrows).
Blood tests showed prothrombin time of 15.7s and activated partial thromboplastin time (aPTT) of > 180s, worse-ning kidney function (creatinine 1.8 mg/dL and urea 84 mg/dL) with new-onset glomerular hematuria and proteinuria of 10.5 g/24h. His hemoglobin was 11 g/dL and platelets were 224.000 cel/mm3. Peripheral blood smear showed anisocytosis and poikilocytosis. He had a C-reactive protein of 1,84 g/dL, positive IgM anticardiolipin (49 U/L, reference range < 40U/L) without other antiphospholipid antibodies (aPL), positive antinuclear antibodies (ANA) (1/1280 with homogeneous nuclear staining), elevated beta2 microglobulin (5.66 ng/mL), low albumin (2.9 g/dL), no complement consumption and negative anti-neutrophil cytoplasmic antibodies. No monoclonal gammopathy was detected. Human immunodeficiency virus, hepatitis B and C serologies were negative. The coagulation panel showed low fibrinogen (182 mg/dL), elevated D-dimer levels (28.52 mcg/mL) and persistent elevation of aPTT that normalized after mixing with normal plasma. Von Willebrand factor activity and antigen were normal, factor VII, IX, X and XI were elevated but factor XII level was of 25%. Upper digestive endoscopy was per-formed and showed gastropathy of body and antrum with diffuse hyperemia and hematinic stains, for which oral proton pump inhibitor was started.
The diagnosis of concomitant congenital factor XII deficiency and DIC was assumed. Brain, skin, gastric and renal manifestations were attributed to microthrombotic phenomena due to the latter. Several investigations were performed to find the underlying cause. Holter showed no rhythm disturbance. Transthoracic echocardiography detected a moderate aortic stenosis, a dilated left auricle, mild pulmonary hypertension and a small pericardial effusion. Additionally, transesophageal echocardiography showed aortic atheromatosis, with no high-risk embolic sources. Body computed tomography (CT) showed bilateral pleural effusion, centrilobular emphysema, and multiple mediastinal and hilar adenopathies (Fig. 2).
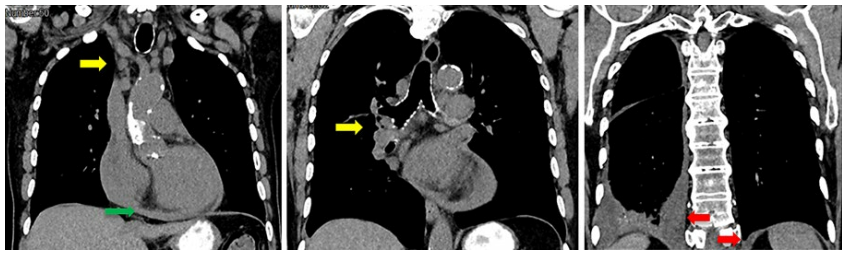
Figure 2: Chest CT showing bilateral pleural effusion (red arrows), pericardial effusion (green arrow) and multiple mediastinal and hilar adenopathies (yellow arrows).
During hospitalization fibrinogen and platelets progressively decreased and D-dimer levels increased, with worsening of renal function. He maintained mild anemia with aspartate aminotransferase and lactate dehydrogenase slightly elevated, without schistocytes on serial peripheral blood smears.
Assuming continued microthrombosis, therapeutic dose enoxaparin was started, with improvement of coagulation parameters and stabilization of neurological deficits and kid-ney function.
He underwent a bone marrow biopsy and aspirate which showed no pathological findings. We then proceeded with mediastinal lymph node excision through mediastinoscopy that revealed adenocarcinoma metastases with numerous lymphovascular invasions. Cells stained positive for cytoke-ratin 7, and thyroid transcription factor-1 with no cytokeratin 20 expression, which was compatible with primary pulmonary origin (Fig. 3).
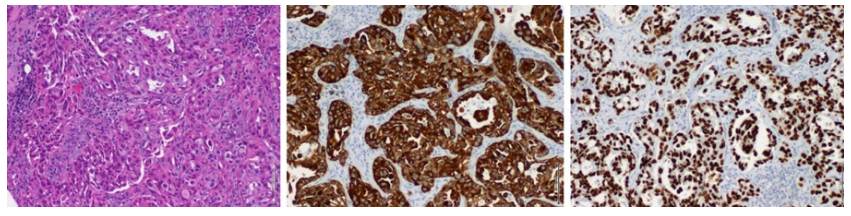
Figure 3: Histopathology of lymph node biopsy on hematoxylineosin (on the left), cytokeratin 7 immunostaining (center image) and thyroid transcription factor-1 staining (on the right), suggesting pulmonary adenocarcinoma.
The clinical picture stabilized enough for the patient to be discharged waiting for the histologic results. Four days later, the patient was readmitted to another hospital with mental confusion and respiratory distress. Cranial CT showed recent ischemic lesions in the right temporo-insular, parietal, and frontal topography, and hypodense focal areas in the left fronto-insular and cerebellar regions. Enoxaparin was suspended and antibiotics for nosocomial pneumonia were started. He deteriorated with worsening encephalopathy, thrombocytopenia and renal dysfunction leading to death within a week.
Discussion
Although there are signs of a hypercoagulable state in virtually all patients with advanced cancer, DIC seems to be much less frequent. The incidence in patients with solid tumors was approximately 7% in several clinical studies, which is inferior to venous thromboembolism occurrence.4 A study of 716 subjects with lung cancer identified a venous or arterial thromboembolic event in 2.2%, but DIC only in 0.7% patients at diagnosis.5
DIC was defined by the International Society on Thrombosis and Haemostasis as «an acquired syndrome characterized by intravascular activation of coagulation with a loss of localization arising from different causes». Malignancies are the most common underlying condition.6
Clinical scenarios range from subclinical to overt DIC, presenting either acutely or chronically with thrombosis, bleeding or both.1,6Manifestations depend on the balance between the clot formation and organ’s ability to compensate for the ongoing consumption of platelets and clotting proteins.6 Specifically in malignancy-associated DIC, three forms can be distinguished: (a) procoagulant, which manifests by micro and macrovascular thrombosis, (b) hyperfibrinolytic, and (c) subclinical, where the amounts of thrombin generated do not cause clinically obvious thrombosis, but abnormalities in laboratory markers of coagulation or fibrinolysis can be seen.7 The risk of thrombosis is especially high for mucinsecreting tumors such as lung adenocarcinomas,1 which are associated with procoagulant or subclinical forms of DIC.4,6,7Even in the setting of procoagulant DIC, arterial thromboembolism is far less common than venous events.4,7Specifically, ischemic stroke as the first manifestation is rare and is associated with advanced unrecognized disease.3,5
Our patient presented with evidence of multifocal cerebral, gastric, renal and skin ischemia with near normal hemoglobin and platelets but low fibrinogen and elevated D-dimers, suggestive of underlying microthrombosis. Since clot formation primarily involves the microvessels, presentation with organ failure without overt thrombosis is possible, with renal dysfunction being the most common type.6 Our patient also presented with diffuse gastropathy and hematinic stains, that can translate gastric ischemia, a reported manifestation of DIC.8 Differential diagnosis includes other thrombotic microangiopathies (TMA), such as thrombotic thrombocytopenic purpura and heparin-induced thrombocytopenia, but our patient had no suggestive features of these entities. Anti-platelet factor 4 antibodies were not ordered because the 4T score was low. Despite the nephrotic range proteinuria and low serum albumin there was no marked edema, and we assumed these changes in the light of the underlying DIC. Since coagulation abnormalities prevailed, we assumed a DIC process progressing from subclinical to an overt procoagulant form, presenting as ischemic stroke. Markedly elevated D-dimers were a major clue to the diagnosis. In fact, D-dimers act as a surrogate marker for excess thrombin generation and fibrinolysis, being useful in establishing the diagnosis and monitoring the disease.7 However, since there are no specific findings, the diagnosis of DIC can be challenging, especially when it is the first manifestation of a previously unrecognized condition.
Our patient had several confounding factors. First, there was a disproportionate elevation of aPTT without hemorrhagic phenomena that was not attributable to DIC. Mixing tests allowed the correction of aPTT, therefore excluding the presence of coagulation inhibitors. Then, dosing of factors of the intrinsic pathway confirmed factor XII deficiency, which is a rare autosomal recessive disorder, causing elevated isolated aPTT without increased bleeding risk.10,11We assumed this as a coincidental finding with no clinical consequences.
Second, the immunological phenomena manifested by a high titer ANA with evidence of polyserositis but no other features suggestive of auto-immune disorder. ANAs have long been associated with malignancy, classically considered epiphenomena related to the release of tumor neoantigens.12,13ANAs with antinucleolar patterns have been described in association with lung cancer12 and we interpreted them in this setting.
Finally, our patient had positive IgM anticardiolipin autoantibodies. aPL are increasingly described in association with malignancies, and lupus anticoagulant and anticardiolipin antibodies have been specifically shown in lung cancer.13,14Whether they add to the thrombotic risk in these patients is more controversial.13,14Since anticardiolipin IgM was isolated and present in a low titer, it is difficult to ascertain if it had some, if any, contribution to the thrombotic events.
DIC management consists of supportive measures and treatment of the underlying cause.4,6In procoagulant or sub-clinical forms anticoagulation should be instituted to restore organ perfusion, alleviate symptoms, and prevent subsequent organ damage.3,6Low-molecular-weight heparin or direct anticoagulants can be used.7 Uncontrolled studies have shown that low-molecular-weight heparin can improve laboratory abnormalities caused by DIC,4 as seen in our case.
The occurrence of DIC is an independent poor prognostic factor in patients with solid tumors.5 In lung cancer, the mortality rate is high, with lack or failure of directed therapy resulting in extremely short survival, of 13 days on average,5,8as occurred in our patient.
Conclusion
In summary, we describe an unusual clinical case of ischemic stroke as the first manifestation of DIC, in the setting of occult lung adenocarcinoma. There was additional evidence of thrombosis affecting at the kidney, the skin and stomach, with accompanying immunological phenomena. DIC should be always considered in the differential diagnosis of patients with multiple site thrombosis and trigger the investigation of the underlying condition.















