Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT-2 inhibitors) prevent the reabsorption of glucose in the proximal renal tubule, resulting in renal excretion of glucose and subsequent reduction in serum levels. They have demonstrated evidence in decreasing cardiovascular complications, mortality, and the risk of developing end-stage renal disease in patients with type 2 diabetes mellitus (T2DM).1-3Several adverse effects have been described, but none of these agents have been associated with liver enzyme elevation, and since their approval, there have only been rare and inconclusive reports of drug-induced liver injury (DILI) associated with this class.2,4The authors present a rare case of a diabetic patient who developed DILI after increasing the dose of dapagliflozin.
Case Report
A 71-year-old woman presented with asthenia, anorexia, nausea, and abdominal pain for approximately 1 month. She had a history of T2DM and was taking metformin/dapagliflozin 1000/5 mg twice daily for at least 3 years (increased to three times daily two months prior), indapamide 1.5 mg/day, olmesartan 20 mg/day, amlodipine 5 mg/day for hypertension, and atorvastatin 20 mg/day for dyslipidemia. The patient denied alcohol, drug, or toxic substance consumption. On physical examination, she had jaundice and tenderness on abdominal palpation, without other noteworthy findings. Laboratory tests showed elevated markers of hepatocellular injury and cholestasis (aminotransferases >10 times the upper limit of normal (ULN), alkaline phosphatase and gamma-glutamyl transferase >8 and 46 times the ULN, respectively, and total bilirubin of 4.30 mg/dL, predominantly direct bilirubin). Inflammatory parameters were not elevated. Liver enzymes were normal before the increase in dapagliflozin dose, and the other medications had been part of her regular therapy for a long time. Thoracoabdominopelvic computed tomography revealed hepatic steatosis without other abnormalities. The patient was admitted with acute hepatitis, and all medications, including dapagliflozin, were discontinued.
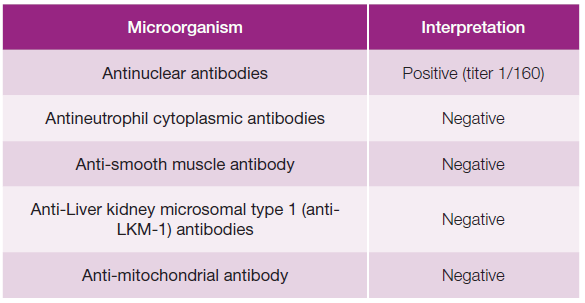
Blood cultures and serologies for hepatotropic pathogens were negative (Table 1). Autoimmune hepatitis was excluded, and no other relevant immunological alterations were identified (Table 2). There was no evidence of excess copper or iron. Magnetic resonance cholangiopancreatography did not reveal any abnormalities, including biliary obstruction. Based on the findings, DILI secondary to dapagliflozin became the most likely hypothesis. Liver biopsy showed trabecular architecture with portal fibrosis (moderate) and hepatitis lesions (mild), which, although nonspecific, could indicate a toxic/drug etiology (Fig. 1). Given the temporal relationship with the increase in dapagliflozin dose, the toxicity was attributed to this medication. The patient had a favorable outcome during hospitalization (Table 3; Fig. 2). One month after discharge, she was asymptomatic with normalization of liver enzymes (Table 3; Fig. 2) and had resumed her previous medications (corresponding to D47 in Table 3), except for dapagliflozin, which was replaced with another class of antidiabetic agents (semaglutide and metformin), maintaining normal liver enzymes for over 2 months after reintroducing all medication (corresponding to D119 in Table 3). The RUCAM (Roussel Uclaf Causality Assessment Method), which is a well-established tool in common use to quantitatively assess causality in cases of suspected DILI,5was applied and the obtained result (total score of 8 points) suggests a probable degree of causality for the drug in question.
Table 1: Blood serologies (hepatotropic virus and bacteria).
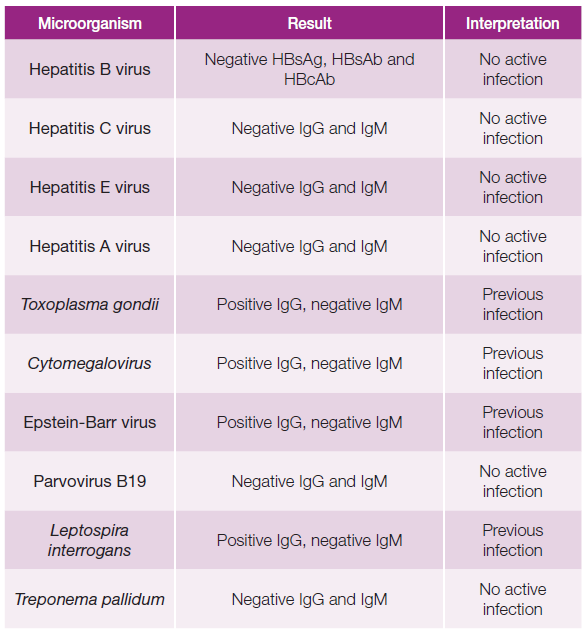
IgG - immunoglobulin G; IgM - immunoglobulin M; HBsAg - hepatitis B surface antigen; HBsAb - hepatitis B surface antibody; HBcAb - hepatitis B core anti-body; Ag- antigen; Ab - antibody.
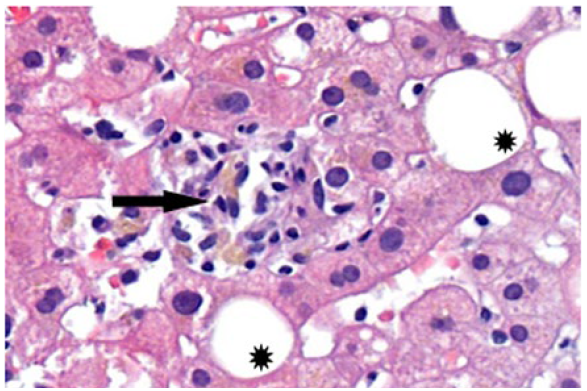
Figure 1: Liver parenchyma with Intralobular necro-inflammatory focus (arrow) and focal lesions of steatosis (stars).
Table 3: Liver enzymes evolving pattern.
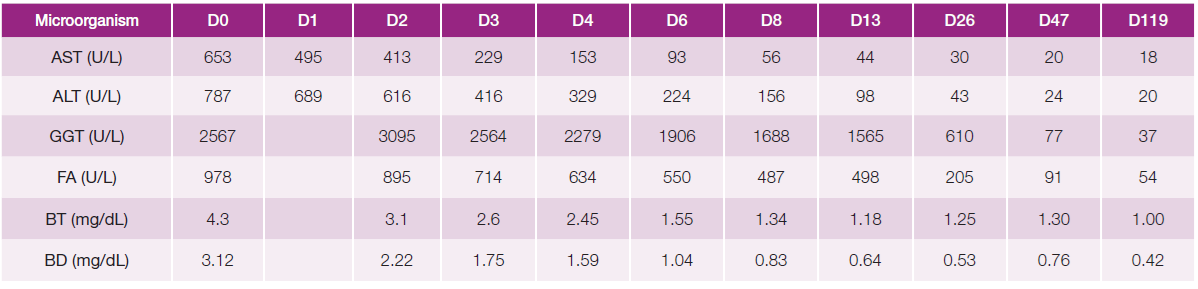
AST - aspartate aminotransferase. ALT - alanine aminotransferase. GGT - gamma - glutamyl transferase, FA - alkaline phosphatase, BT - total bilirubin, BD - direct bilirubin.
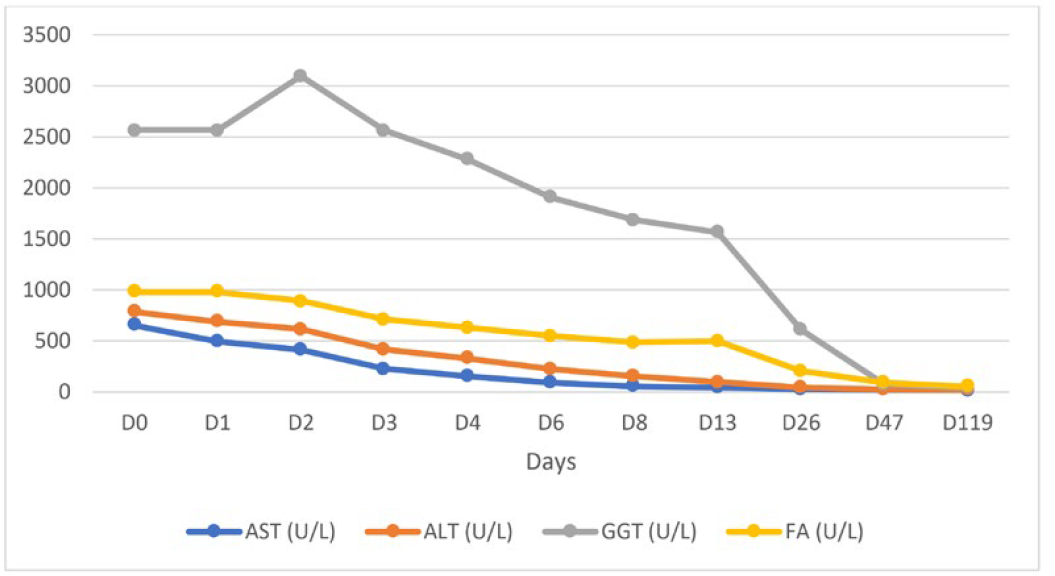
Figure 2: Liver enzymes evolving pattern: progressive normalization of liver enzymes during the hospitalization (13 days); after discharge (D47 - one month after discharge there was a complete normalization of the hepatic alterations) and 2 months after reintroducting all medication (D119), liver enzymes remain normal.
Discussion
DILI is a challenging clinical condition in terms of both diagnosis and treatment, and it is the most common cause of acute liver failure in developed countries.6,7It presents a wide range of clinical manifestations, and identification of the implicated substance is crucial.4 Since DILI can be induced by many medications and dietary supplements, it can represent a diagnostic challenge, particularly in polymedicated patients, and is mainly based on the exclusion of other causes.1,6In several large randomized clinical trials, dapagliflozin was not associated with liver enzyme elevation during treatment.2 In fact, retrospective studies have shown that treatment with SGLT2 inhibitors was associated with a decrease in alanine aminotransferase levels, likely due to concomitant improvement in hepatic steatosis resulting from optimized glycemic control or weight loss, or both.2 Since its approval and wider use, at least three reports of liver injury possibly associated with an SGLT2 inhibitor have been published, two of which were in patients with pre-established chronic liver disease.1,2,8 The pathogenesis of DILI secondary to dapagliflozin may be related to an idiosyncratic reaction triggered by dysregulated immune response, alteration of dapagliflozin metabolic pathways, or both.8 There is no specific biomarker capable of associating with SGLT2 toxicity.8 However, when a temporal relationship is observed between drug administration and the onset of hepatic alterations, the diagnosis becomes more evident, and the resolution of these alterations upon drug discontinuation favors the diagnosis of DILI.6 However, this diagnosis is not always evident, especially when it involves a substance for which there is no clear evidence of its association with liver injury.1 Liver biopsy can support the diagnosis of DILI through the histological pattern, which, along with the clinical history, laboratory data, and imaging exams, can corroborate the diagnosis after other causes have been excluded.1,6However, it is necessary to consider that in polymedicated patients, concomitant drugs can affect susceptibility to DILI through drug interactions, either by modulating the metabolism of other drugs through induction, inhibition, or substrate competition, particularly in cytochrome reactions.8 This interaction can result in the hepatotoxic potential of a drug that, on its own, would not cause clinically relevant DILI.8 In this case, among the concomitant drugs that the patient was taking, statin seems to have the highest potential for modulating DILI.8 However, previous studies have shown a protective effect of statins against the progression to acute liver failure.8Therefore, it is difficult to determine the true modulating role of concomitant drugs, but it is important to always consider their possible interaction. Confirmation of the DILI diagnosis requires patient follow-up and evaluation of clinical and laboratory evolution over time after drug withdrawal,1 despite this, it cannot be fully established, and there is always some degree of doubt regarding the causality link, even if no other cause has been found. However, re-exposure for confirmation would not be ethically acceptable.
Conclusion
DILI associated with SGLT2 inhibitors is very rare, and although it has never been associated with acute liver failure or chronic hepatitis,2 the authors aim to raise awareness about the potential hepatotoxic effects of dapagliflozin, emphasizing the importance of early diagnosis and appropriate treatment, including immediate discontinuation of the drug.