One of the major challenges in primary care and hospital medicine lies on the efficient management of patients with localised prostate cancer. Active surveillance is a monitoring strategy recommended for men with localised and low-risk disease (T1/2, Gleason score ≤ 6 and prostate-specific antigen (PSA) ≤10). This approach involves regular PSA measurements and repeat biopsy sampling. Magnetic resonance imaging might be considered in patients whose PSA levels have increased.1 Among men treated with definitive radiation therapy, the National Comprehensive Cancer Network recommends testing prostate-specific antigen (PSA) every 6 to 12 months for 5 years and annually thereafter, while the American Urology Association recommends testing for at least 10 years, with the frequency to be determined by the risk of relapse and patient preferences.2 As one of the cancer leading causes of death worldwide, monitoring prostate cancer after treatment is crucial for detecting any signs of recurrence and managing potential side effects of treatment.3Despite most cases present with localized diseased and being correlated with favourable outcome,3 20%-50% will experience biochemical recurrence within 10 years.4 The main indicators used to assess patient’s prognosis and risk of recurrence include PSA baseline level, PSA doubling time, tumour stage, age, comorbidities and pathologic features such as Gleason score, extracapsular extension, seminal vesicle involvement, surgical margin and lymph node status.5 Nearly 20% of men are diagnosed with metastatic disease, with a marked predilection for lymph nodes and bones.3 The occurrence of peritoneal carcinomatosis leading to malignant ascites in recurrent prostate cancer is uncommon.6,7As a matter of fact, the emergence of ascites secondary to prostate cancer can go unnoticed to many physicians. Herein we describe a case of a 64-year-old man diagnosed with localised prostate cancer after a PSA screening and transrectal ultrasound guided prostate biopsy. Histopathology examination revealed a poorly differentiated adenocarcinoma (Gleason score of 6) within the left lobe (stage T1cN0M0). As a result, he was submitted to a 7-week program of external radiation therapy. During the subsequent follow-up, levels of serum PSA decreased from 14.96 ng/L (normal value < 4 ng/L) to 1.1 ng/L within 2 years. At that time, he was discharged from his urologist appointments. The patient had no subsequent consultations and did not seek further medical care. After thirteen years, he presented to the hospital's emergency department with abdominal distension and dyspnea. An abdominal ultra-sound showed a large-free peritoneal effusion, and the PSA level was increased by 50-fold (225.8 ng/mL). A therapeutic and diagnostic paracentesis was conducted, draining 7 L of hemorrhagic ascitic fluid with a serum-ascites albumin gradient of 0.6 g/dL (indicating absence of portal hypertension), but no malignant cells. The ascitic fluid cell count showed an increased proportion of mononuclear cells. The levels of lactate dehydrogenase, adenosine deaminase, amylase, and glucose in the ascitic fluid were within normal limits and bacterial culture was negative. These results ruled out spontaneous bacterial peritonitis and tuberculosis. Considering the high suspicion of peritoneal carcinomatosis and tumour progression a positron emission tomography/computed tomography (PET/CT) scan using gallium-68 prostate-specific membrane antigen (68Ga-PSMA) was performed for tumour staging. The results showed a locally advanced prostate cancer with peritoneal carcinomatosis, peritoneal effusion in the subhepatic space, subphrenic space and rectovesical pouch and bone metastasis (Fig. 1). The patient underwent a second prostate biopsy, with histopathological findings (Fig. 2) revealing a poorly differentiated Gleason score of 8 in 70% of the biopsy in the right lobe and 80% of the biopsy in the left lobe - grade group 4 (WHO, 2016).8 Bicalutamide and leuprorelin were started at this juncture. However, the patient's overall condition rapidly deteriorated. He was referred for palliative care and died within four months of starting medication.
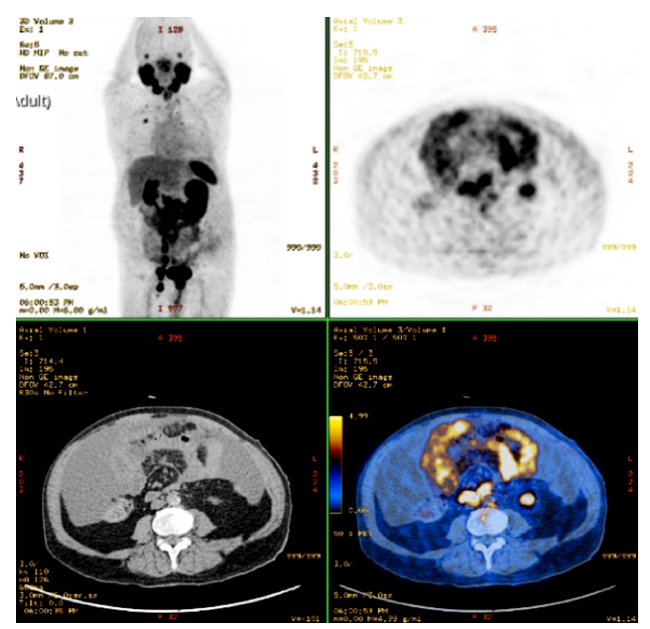
Figure 1: PET/CT with 68Ga-PSMA: The prostate shows high absorption without clear separation from the rectum, peritoneal effusion, scattered peritoneal thickening with heterogeneous uptake and multiple bilateral abdominopelvic adenopathies.
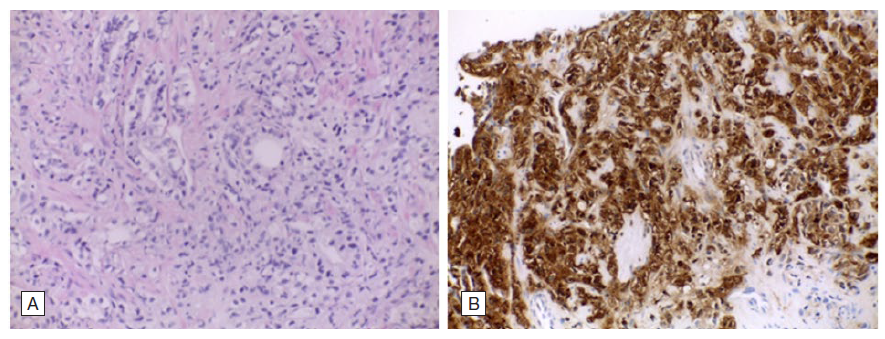
Figure 2: Histopathological findings from the second prostate biopsy: A - Prostate poorly differentiated acinar adenocarcinoma with neoplastic cells organized in trabeculae, fused and malformed glands, Gleason 8 (4+4), HE, 200x. B - PSAP (prostatic specific acid phosphatase) intense (3+) immunohistochemical expression in the neoplastic cells, PSAP, 200x.
This case highlights the importance of consistent medical check-ups and the role of sustained monitoring in managing prostate cancer effectively. Post-treatment monitoring for prostate cancer is essential to ensure early detection of recurrence and manage any long-term side effects. It involves regular PSA testing, clinical evaluations, and sometimes imaging studies, depending on individual risk factors and the initial treatment received. The ultimate goal is to maintain quality of life while promptly addressing any signs of cancer recurrence.















