Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.19 no.1 Lisboa jan. 2012
Celiac disease and upper tract Crohns disease – A rare association
Sara Folgado Alberto*, Alexandra Martins, João Ramos de Deus
Serviço de Gastroenterologia, Hospital Fernando Fonseca
*Autor para correspondência
ABSTRACT
The authors present the case of a 47 years old female, with no past or familial history, hospitalized due to vomiting and significant weight loss over the last 9 months, without abdominal pain, blood loss, constipation or diarrhoea. Blood tests results revealed iron deficiency anaemia, seropositivity for ASCA, anti-transglutaminase antibody and HLA-DQ2.
Upper GI endoscopy showed several ulcers in duodenal mucosa and an ulcerated stricture in D3; biopsies were taken and histopathology revealed transmural inflammatory chronic infiltrate, crypt loss and some areas of villous atrophy and intraepithelial lymphocytosis. The small bowel follow through confirmed 2 main strictures in duodenum and proximal jejunum. She was started on prednisolone, azathioprine and a gluten-free diet with an initial good response although relapse was detected one month later. In this last admission, parenteral nutrition was necessary due to her deteriorated nutritional state and she also started infliximab, with subsequent significant clinical and endoscopic improvement.
KEY-WORDS: Crohns disease, celiac disease, association, small bowel stricture, infliximab
Doença celíaca e doença de Crohn do tubo digestivo superior – Uma rara associação
RESUMO
Os autores apresentam o caso clínico de uma mulher de 47 anos, sem antecedentes pessoais ou familiares, internada por vómitos e emagrecimento significativo nos últimos 9 meses; sem dor abdominal, perdas hemáticas, obstipação ou diarreia. Laboratorialmente, de destacar anemia ferropénica, seropositividade para ASCA, anticorpo anti-transglutaminase e para HLA-DQ2.
A endoscopia digestiva alta mostrou várias úlceras na mucosa duodenal e estenose ulcerada em D3, cuja histologia revelou infiltrado inflamatório crónico transmural, atrofia de criptas e áreas com atrofia vilositária e linfocitose intra-epitelial.
O estudo baritado do intestino delgado confirmou a presença de 2 estenoses no duodeno e jejuno proximal. A doente foi medicada com prednisolona, azatioprina e dieta sem glúten, inicialmente com boa resposta, mas com recidiva após 1 mês. Foi readmitida, com necessidade de alimentação parentérica devido ao mau estado nutricional. Começou infliximab, com melhoria clínica e endoscópica subsequente.
PALAVRAS-CHAVE: Doença celiaca, doença de Crohn, associação, estenose do intestino delgado, infliximab
INTRODUCTION
Both Crohns and Celiac diseases are chronic and imune diseases of the small bowel that share some features.
Celiac disease is a small bowel disorder with mucosal inflammation, crypt hyperplasia and villous atrophy, induced by gluten ingestion in genetically predisposed individuals. Crohns disease is a chronic inflammatory immunomediated disease potentially affecting the entire digestive system but with predilection sites in the terminal ileum and colon1, 2.
Some authors suggest that they may be related to each other, although there are only a few reports in literature concerning their simultaneous occurrence in the same patient1,2.
CASE REPORT
A 47 years old caucasian woman, without personals or familiar priors, was admitted in our department due to nausea, vomiting and weight loss of 35% in the last 9 months. She had no abdominal pain or blood loss and her bowel habits remained regular, without constipation or diarrhea. The physical examination only showed a thin woman, weighting 35 Kg, body mass index of 15, with digital hypocratism.
The initial blood samples revealed: iron deficiency anaemia (haemoglobin:10g/dl; mean corpuscular volume:73fl; iron:27 μg/dL; transferrin: 192 μg/dL; ferritin:50 ng/dL), sedimentation rate: 55mm; C reactive protein: 11.6 mg/dL; albumin: 2.1 mg/dL. Cultures from blood and faeces, Human Immunodeficiency Virus, immunoglobulin profile and Mantoux test were either negative or normal.
An upper endoscopy was performed showing gastric stasis, hyperemia and multiple aftoid ulcers in the duodenum and a stricture in D3 with ulcerated mucosa (Figures 1, 2 and 3).
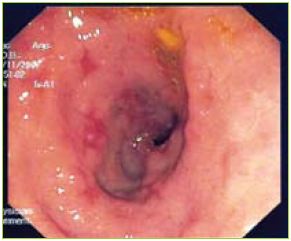
Figure 1 Multiple aftoid ulcers in the duodenum
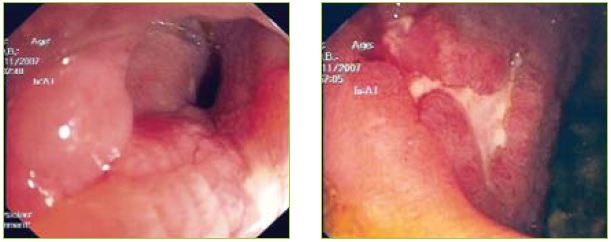
Figures 2 and 3 Multiple aftoid ulcers in the duodenum and a stricture in D3 with ulcerated mucosa.
The biopsies of the aftoid ulcers and ulcerated stricture revealed crypt loss and transmural chronic inflammatory infiltrate without granulomas. In areas of normal mucosa, some villous atrophy and intra-epithelial lymphocytosis were observed (Figure 4). Cytomegalovirus was excluded by immunohistochemistry techniques.
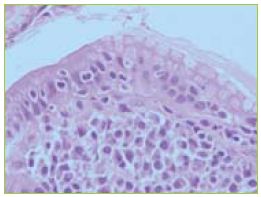
Figure 4 In the areas of normal mucosa the histology revealed villous atrophy and intra-epithelial lymphocytosis.
She started ciprofloxacin and metronidazol to rule out bacterial proliferation that was stopped later on. A normal ileocolonoscopy with normal biopsies was also performed as well as a small bowel follow through. In this last exam two main strictures with irregular mucosa were observed, one in the duodenum and the other in the proximal jejunum.
Abdominal ultrasound and computed tomography showed small mesenteric lymphadenopathies and densification of adipose tissue and mesentery.
This time, new blood samples revealed positive serology for celiac disease (anti tissue transglutaminase IgG and IgA) and positive anti Saccharomyces cerevisiae (ASCA). The HLA-DQ2 was also positive.
We assumed that the patient had Crohns disease (the aftoid ulcers were typical, there was a stricture and densification of adipose tissue in CT scan and a positive ASCA) and also a celiac disease (positive and specific serology and intraepithelial lymphocytosis). Lymphoproliferative disease was excluded by repeated negative histology and also by a normal immunohistochemistry profile of the mucosal lymphocytes without any clonal proliferation.
She was put on a gluten free diet, prednisolone 40 mg/ daily (with progressive tapering after two weeks) and azathioprine (2,5 mg/kg/d) due to the severity of her symptoms. She was discharged with clinical improvement. About one month later she was readmitted with the same complaints, after intake of diclofenac for back pain and poor adherence to a gluten-free diet.
Infectious complications, such as intraabdominal abscess, were ruled out. An enteroscopy revealed the same lesions, persisting aftoid ulcers and D3 stricture. Due to her deteriorated nutrional state, both enteral (gluten-free) and parenteral nutrition were undertaken for almost 3 weeks, with a good clinical response. By this time, 10 weeks after the beginning of azathioprine, another enteroscopy was performed showing a duodenal mucosa in healing process with a significant improvement of the stricture with no need for endoscopic dilation. A small bowel follow through was repeated revealing the persistence of the two main strictures. We decided to maintain azathioprine and start infliximab (0, 2 and 6 weeks and then maintenance q8 weeks). The patient remained symptom free and gained 8 Kg in a 6 months period.
DISCUSSION
We presented the clinical case of a patient with Crohns and celiac disease.
Both are genetically predisposed diseases that share a similar immune-mediated Th1 inflammatory cascade in response to loss of tolerance to external antigens (glúten for celiac disease and intestinal flora to Crohns disease)1,2.
This may be responsible for the concomitant occurrence of both diseases. In several studies the association with ulcerative colitis is almost absent, probably because the last one has a Th2 pattern1, although some studies related exactly the contrary3.
Celiac patients and their relatives have a greater predisposition to Crohns disease compared to the control population1,2,4, with equal predisposition to other autoimmune diseases such as insulin-dependent diabetes mellitus or thyroiditis2. In contrast, the prevalence of celiac disease among inflammatory bowel disease (IBD) patients was comparable with general population5.
Moreover, the auto antibody profile of both diseases may be crossed. Anti-tissue transglutaminase antibody has high sensitivity and specificity for celiac disease6. Despite the high specificity of ASCA for Crohns disease, they can also be positive in some patients with celiac disease7.
In spite of these explanations and the relatively high prevalence of both diseases, there are only a few reports describing the association between Crohns and celiac disease1,2,8,9,10.
In the majority of the published cases, Celiac disease preceded the diagnosis of Crohns disease2,8,9. Furthermore, most patients had symptomatic Celiac disease2 and colonic or terminal ileum Crohns disease2. We think that our patient fits the rare profile of a latent celiac with an active upper tract Crohns disease with stricturing behaviour (A3, B2, L4 according to Montreal s classification).
The use of azathioprine and infliximab is recommended in both diseases when they are too severe or refractory11. Despite the latent celiac disease pattern, we are convinced that its reasonable to continue a gluten-free diet, even lifelong, to prevent relapses.
CONCLUSION
The occurrence of celiac disease and upper Crohns disease in the same patient is an extremely rare association, although they share some features as previously described. By having both conditions affecting the duodenum and proximal jejunum, the nutritional status of this patient was severely compromised, demanding a challenging treatment strategy, with step-up drug therapy. Because this was successful, we prevented more aggressive therapy, such as endoscopic dilation or surgery.
REFERENCES
1. Masachs M, Casellas F, Malagelada R. Inflammatory bowel disease in celiac patients. Rev Esp Enferm Dig 2007;99:446-450. [ Links ]
2. Schedel J, Rockmann F, Bongartz T, et al. Association of crohns disease and latent celiac disease: a case report and review of the literature. Int J Colorectal Dis 2005;20:376-380. [ Links ]
3. Cottone M, Marrone C, Casà A, et al. Familiar occurrence of inflammatory bowel disease in celiac disease. Inflamm Bowel Dis 2003;9:321-323. [ Links ]
4. Yang A, Chen Y, Scherl E, et al. Inflammatory bowel disease in patients with celiac disease. Inflamm Bowel Dis 2005; 11:528-532. [ Links ]
5. Leeds J, Horoldt B, Sidhu R, et al. Is there an association between celiac disease and inflammatory bowel diseases? A study of relative prevalence in comparison with population controls. Scand J Gastroenterol 2007;42:1214-1220. [ Links ]
6. Di Tola M, Sabbatella L, Anania MC, et al. Anti-tissue transglutaminase antibodies in inflammatory bowel disease : new evidence. Clin Chem Lab Med 2004;42:1092-1097. [ Links ]
7. Barta Z, Csípõ I, Szabó GG, et al. Seroreactivity against saccharomyces cerevisiae in patients with crohns disease and celiac disease. World J Gastroenterol 2003;9:2308-2312. [ Links ]
8. Kitis G, Holmes GK, Cooper BT, et al. Association of celiac disease and inflammatory bowel disease. Gut 1980;21:636-641. [ Links ]
9. Curtis WD, Schuman BM, Griffin JW. Association of gluten-sensitive enteropathy and crohns colitis. Am J Gastroenterol 1992;87:1634-1637. [ Links ]
10. Karoui S, Boubaker J, Hamzaoui S, et al. Association of asymptomatic celiac disease and crohns disease (article in french). Ann Med Interne (Paris) 2000;151:411-412. [ Links ]
11. Gillett H, Arnott ID, McIntyre M, et al. Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology 2002;122:800-805. [ Links ]
*Autor para correspondência
Address: IC-19, Venteira, 2720-276 Amadora, Portugal
Mobile: +351 964 077 817
E-mail: sarafalberto@gmail.com
Recebido para publicação: 08/12/2009 e Aceite para publicação: 20/05/2010













