Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.19 no.4 Lisboa jul. 2012
Portuguese consensus on the best practice for the management of inflammatory bowel disease: IBD ahead 2010 meeting results
Consenso português sobre a melhor prática clínica para tratamento da Doença Inflamatória do Intestino: resultados da reunião IBD Ahead 2010
Fernando Magroa,b,∗, Marilia Cravoc, Paula Lagod, Paula Ministroe, Paula Peixef, Francisco Portelag, Jaime Ramosh, Lourdes Tavaresc , IBD ahead 2010 group
a Department of Pharmacology and Therapeutics, Faculdade de Medicina, Universidade do Porto, Portugal
b Department of Gastrenterology, Hospital de São João, Porto, Portugal
c Department of Gastrenterology, Centro Hospitalar Lisboa Norte - Hospital de Santa Maria, Lisboa, Portugal
d Department of Gastrenterology, Centro Hospitalar do Porto - Hospital Geral de Santo António, Porto, Portugal
e Department of Gastrenterology, Hospital de São Teotónio, Viseu, Portugal
f Department of Gastrenterology, Centro Hospitalar Lisboa Ocidental - Hospital Egas Moniz, Lisboa, Portugal
g Department of Gastrenterology, Hospitais da Universidade de Coimbra, Coimbra, Portugal
h Department of Gastrenterology, Centro Hospitalar Lisboa Central, Lisboa, Portugal
*Corresponding author
Abstract
Introduction: The treatment of inflammatory bowel disease (IBD) has focussed on the management of symptoms but is becoming more resolute on changing the course of the disease and its complications in the long-term. In order to minimize the development of complications and to improve outcomes for these patients it is important to develop other strategies to manage IBD and to optimize current clinical practice.
Objective: This article reports the main consensus statements reached during the Portuguese National Meeting on improvement of disease control in IBD, on optimization of corticosteroid and immunosuppressive use in Crohns disease and on best practice in topics of current interest in Crohns disease.
Methods: An International Steering Committee selected the top 10 most important unanswered practical questions on the use of conventional therapy in Crohns disease, to be debated and analysed in several National Meetings of different countries. In each country a National Steering Committee (NSC) was created to moderate a National Meeting during which several expert groups answered the selected questions in light of their clinical practice. Answers were classified according to the Oxford levels of evidence.
Consensus: A general consensus was obtained, some of the conclusions were as follows. It is important to introduce conventional corticosteroids in moderate to severely active Crohns disease of any localization with initial duration of treatment varying according to patients response; the best option to prevent steroid-induced side effects is to avoid its prolonged or repetitive use and switching appropriate patients to immunosuppressive therapy. Initiation of immunomodulators early in the disease course should be considered for patients with a poor prognosis and optimal safety monitoring was discussed, with the need to reassess patients at appropriate timepoints, make corticosteroid-free remission a goal and treat beyond symptoms.
Keywords Inflammatory bowel disease; orticosteroids; Crohns disease; Consensus
Resumo
Introdução: O tratamento da Doença Inflamatória Intestinal (DII) tem-se focado no controlo dos sintomas. No entanto, nos últimos anos tem-se vindo a concentrar mais na mudança do curso da doença e das suas complicações a longo-prazo. De forma a minimizar o desenvolvimento de complicações e melhorar a condição dos doentes, torna-se fundamental desenvolver outras estratégias para controlo da DII e optimizar a prática clínica habitual.
Objectivos: Este artigo relata o consenso alcançado por um grupo de peritos durante a reunião Nacional de Peritos relativamente ao controlo da doença e à optimização do uso dos corticosteroides e imunossupressores na doença de Crohn.
Métodos: Uma Comissão Científica Internacional seleccionou as 10 questões práticas mais importantes relativas ao uso da terapêutica convencional na doença de Crohn, afim das mesmas serem debatidas e analisadas em várias Reuniões Nacionais de diversos países. Em cada país foi constituída uma Comissão Científica Nacional para moderar a Reunião Nacional, onde vários peritos nacionais discutiram e responderam às questões colocadas de acordo com a prática clínica. As respostas foram classificadas de acordo com os níveis de evidência de Oxford e avaliadas de acordo com os graus de evidência de Oxford.
Consenso: Foi alcançado um consenso geral. Algumas das conclusões encontradas incluem: a importância da introdução de corticosteroides na doença de Crohn activa moderada a grave de qualquer localização; a duração do tratamento com corticosteroides, nestes casos, deve variar de acordo com a resposta do doente; a melhor opção para prevenir efeitos secundários induzidos pelos corticosteroides é evitar o seu uso prolongado e repetitivo e a passagem apropriada de alguns doentes para terapêutica imunossupressora. A administração de imunomodeladores numa fase inicial do curso da doença deve ser considerada nos doentes com mau prognóstico. Foi também discutida a optimização da monitorização da segurança, com a necessidade de reavaliar os doentes em momentos específicos e apropriados, estipular como objectivo a remissão livre de corticosteroides e o tratamento para além dos sintomas.
Palavras-chave Doença Inflamatória Intestinal; Corticosteroides; Doença de Chron; Consenso
Introduction
Inflammatory bowel disease (IBD) is a chronic idiopathic inflammatory disorder of the gastrointestinal tract which includes Crohns disease and Ulcerative Colitis. Both pathologies are characterized by intermittent presence of symptoms such as abdominal pain, diarrhea, blood in the stool, and systemic symptoms.1
The incidence of IBD is usually higher in subjects between 15 and 30 years of age.2 According to a Portuguese study by Azevedo and co-workers, the incidence of Crohns disease was particularly higher in the age stratum between 17 and 39 years and the prevalence of IBD in Portugal in 2007 was 146 patients per 100,000 subjects, showing na increasing trend between 2003 (when it was 86 patients per 100,000 individuals) and 2007.3 Moreover, the incidence of IBD is considered to be variable in different regions and for different groups of population, and has increased in recent years.3,4 Several studies report that incidence is estimated to be around 5-7 per 100,000 subjects/year for Crohns disease in the northern hemisphere countries, such as the United States of America and northern European countries and about 0.1-4 per 100,000 subjects/year in Southern countries.3,4 In Portugal, according to a study by Shivananda et al., between 1991 and 1993, the estimated incidence of Crohns disease was 2.4 per 100,000 subjects and for Ulcerative colitis it was 2.9 per 100,000.4
The treatment of IBD has focussed on the management of symptoms and, in recent years, has become more resolute on changing the course of the disease and its complications in the long-term. In fact, the probability of developing complications requiring hospitalization and surgery is high and recurrence after surgery is also common.5-7 Therefore, in order to minimize the development of these complications and to improve outcomes for these patients, it is importante to develop other strategies to manage IBD and to optimize current clinical practice.
With the main objectives of discussing ways to improve disease control in IBD, to outline key clinical data and experience leading to optimization of corticosteroid and immunosuppressive use in Crohns disease and to debate the best practice in topics of current interest in Crohns disease, several National Meetings were held in diferente countries. This article reports the main consensus statements reached in the Portuguese National Meeting.
Methodology
Between July and August 2009, 26 key unanswered practical questions on the use of conventional therapy in Crohns disease were identified through market research. During the following months (September and October), 1400 participants from almost 30 countries evaluated those questions through a web-based ranking, giving a higher score for those considered to be the most important. Based on the ranking results, the International Steering Committee selected the top 10 questions to be debated and analysed in several National Meetings of different countries. National Meetings aimed at providing input to ascertain national perspectives to the answers.
The 10 selected questions were:
1. When should we introduce corticosteroids, and for how long?
2. What is the best dosing strategy for the use of corticosteroids in patients with Crohns disease, in terms of: starting and maximum doses, duration, dose escalation/de-escalation (when? rate?), formulation, avoiding side-effects? What duration of corticosteroid treatment is linked to the occurrence of side effects?
3. How early should immunosuppressives be introduced in the management of Crohns disease and which regímen should be used?
4. What is the best dosing strategy for immunosuppressives in Crohns disease, in terms of: starting and maximum doses, duration, dose escalation/deescalation (when? rate?), which immunosuppressive first?
5. How should the efficacy of a treatment be monitored clinically and biologically? What is the definition of treatment failure? When should the effect of treatment be evaluated? Should mucosal healing be assessed?
6. If azathioprine and a biologic are given in combination, should any of the treatments be stopped? Which treatment should be stopped to achieve the smallest reduction in efficacy? When should that treatment be stopped?
7. If the immunosuppressive does not work, what should the approach be? Add steroids? Increase the dosage? Change the immunosuppressive? Move to a biologic?
8. If a patient experiences flare-ups when receiving immunosuppressives or a biologic, should corticosteroids be added?
9. What are the risks of cancers (all kinds) and infections associated with the short-, mid- and long-term use of immunosuppressives and corticosteroids?
10. What is the optimal safety monitoring (clinical, laboratory, radiological) of patients receiving immunosuppressives or corticosteroids? How often?
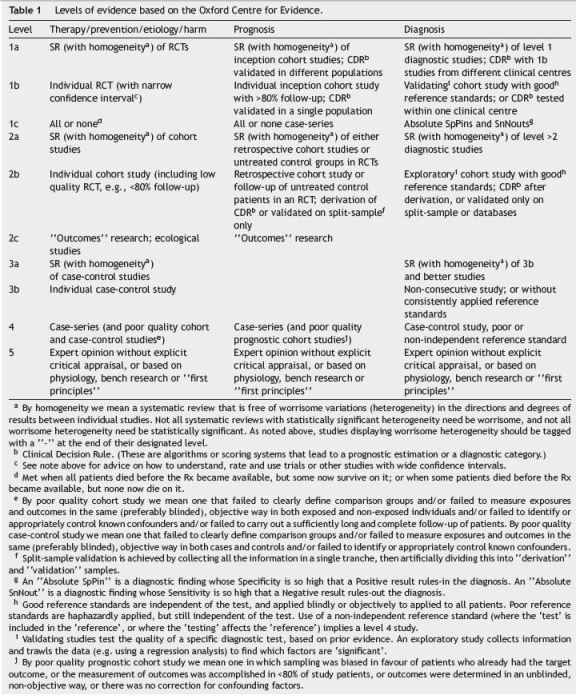
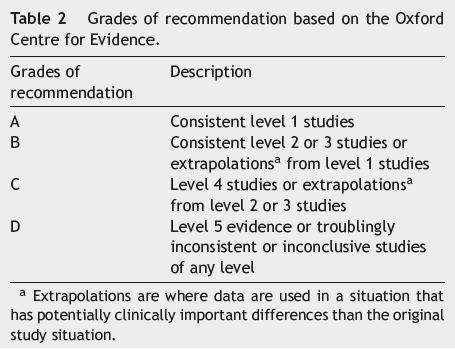
After identifying the 10 questions, a specialist company was contacted to perform a literature search. Based on the literature search, a group of five bibliographic fellows from different countries, analysed the results of the search, and produced a report for each question including draft answers and supporting information with references, based on the evidence levels (Table 1) and grades of recommendation (Table 2) from the Oxford Centre for Evidence.8 The report developed by the bibliographic fellows was reviewed and each of the draft answers was consolidated and approved by a group of project mentors, members of the International Steering Committee.
Methodology of the National Meeting
A National Steering Committee (NSC) was created including eight experts. Their main objective was to help elaborate the agenda, identify additional delegates with good anti- TNF therapy experience, develop/approve materials, and moderate the National Meeting with the end purpose of contributing to the development of its outputs.
During the National Meeting, the 21 participants split into five small groups (Group 1 with five members and the remaining ones with four each) to review two answered questions each. The small groups were chaired by two of the members of the NSC who presented the proposed draft answers and moderated the discussion until the group had agreed on revised wording for the answers to their selected questions. All answers were classified according to the Oxford levels of evidence (Table 1) and graded according to the Oxford grades of evidence (Table 2).8
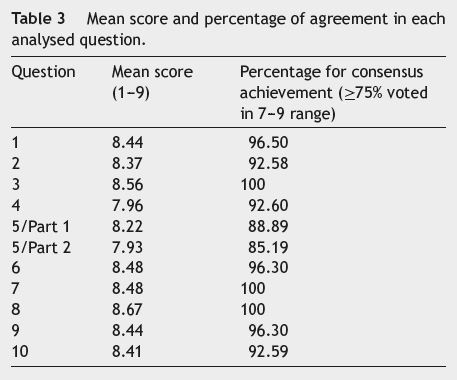
After reaching an agreement, all participants reconvened to present their selected answers to the entire group, followed by an overall group vote to reach a consensus for each answer. If the voting did not achieve an agreement after the initial round, participants discussed the response further and proposed a new answer, one on which an agreement could be reached. If there was no consensus after two votes, there was no further discussion. Participants voted according to a scale from 1 (strong disagreement) to 9 (strong agreement). Consensus was defined as a score of 7-9 by ≥75% of the participants.
Consensus
Table 3 shows the mean scores of agreement and the percentage of participants who agreed with the answer to each question.
Question 1. When should we introduce corticosteroids and for how long?
Draft answer modified by National Meeting Working Group
(1) When considered as a treatment option, conventional corticosteroids should be introduced in moderate to severely active Crohns disease of any localization (level of evidence: 1a; grade of recommendation: A).
(2) However budesonide is indicated in mild active ileocecal and/or right-sided colonic disease and is preferred to conventional corticosteroids in moderately active ileal and right-sided colonic Crohns disease (CDAI up to 300) due to its safety profile (level of evidence: 1a; grade of recommendation: A).
(3) The duration of initial treatment with conventional corticosteroids at full dose might vary depending on the response of the patient. There is no clear evidence that continuing the full dose beyond weeks 1-3 influences remission rates (level of evidence: 2b; grade of recommendation: B).
Question 2. What is the best dosing strategy for the use of corticosteroids in patients with Crohns disease, in terms of: starting and maximum doses, duration, dose escalation/de-escalation (when? rate?), formulation, avoiding side-effects? What duration of corticosteroid treatment is linked to the occurrence of side effects?
Draft answer modified by National Meeting Working Group
(1) The optimal initial dose of conventional corticosteroids in Crohns disease ranges from 40 to 60 mg/day to 1 mg/kg (level of evidence: 2b; grade of recommendation: B).
(2) The optimal starting dose of budesonide is 9 mg/day (level of evidence: 1b; grade of recommendation: A).
(3) The duration of initial treatment with conventional corticosteroids at full dose might vary depending on the response of the patient. There is no clear evidence that continuing the full dose beyond weeks 1-3 influences remission rates (level of evidence: 2b; grade of recommendation: B).
(4) Different duration regimens of budesonide have been utilized, with most using a full dose of 9 mg for 8-10 weeks (level of evidence: 1a/2b; grade of recommendation: A/B).
(5) A tapering regimen of conventional corticosteroids does not seem to influence short- or long-term remission rates (level of evidence: 2b; grade of recommendation: B).
(6) No data are available to allow evaluation of any benefit of intentional escalation of steroid dose (i.e. when steroids have already been started for induction of remission) (level of evidence: 1b/2b; grade of recommendation: A/B).
(7) Neither conventional steroids nor budesonide are effective in maintenance of remission (level of evidence: 1a; grade of recommendation: A).
(8) In corticosteroid-dependent patients with quiescente disease, budesonide may facilitate the withdrawal of conventional corticosteroids, minimizing side-effects before the delayed benefit of azathioprine/ methotrexate in the maintenance of remission (level of evidence: 1b/5; grade of recommendation: A/D).
(9) No data are available on the efficacy of different conventional steroids. Different oral formulations are used for induction of remission in different studies without substantial differences in remission rates (level of evidence: 1b/2b; grade of recommendation: A/B).
(10) IV Corticosteroids are an option for patients failing to respond to oral corticosteroids (level of evidence: 3b/5; grade of recommendation: B/D).
(11) Corticosteroids have been shown to increase the risk of serious and opportunistic infections, both independently and in combination with immunosuppressive and biologic agents. Thus, the best option to prevent steroid-induced side effects is to avoid prolonged or repetitive use and to switch appropriate patients to immunosuppressive therapy. To prevent steroidinduced loss of bone mineral density, calcium and vitamin D supplements should be provided. However, not all steroid-induced side effects are dose- or time-dependent (level of evidence: 2b; grade of recommendation: B).
Question 3. How early should immunosuppressives be introduced in the management of Crohns disease and which regimen should be used?
Draft answer modified by National Meeting Working Group
(1) Initiation of immunosuppressives early in the disease course (at first flare needing steroids) should be considered (level of evidence: 1b; grade of recommendation: A)
(2) This is particularly relevant in patients at high risk of complicated disease: perianal disease, age below 40, extensive disease and structuring or penetrating behaviour at diagnosis (level of evidence: 3b; grade of recommendation: B).
(3) Immunosuppressives are indicated in immunosuppressive-naïve patients starting systemic steroids or infliximab in order to achieve steroid-sparing effects or added benefit (level of evidence: 1b; grade of recommendation: A).
(4) Purine analogues are effective in post-operative prophylaxis immediately after surgical resection of ileocolonic disease (level of evidence: 1a; grade of recommendation: A).
(5) Evidence for the use of purine analogues as first-line therapy in perianal fistulating Crohns disease is limited (level of evidence: 2; grade of recommendation: B).
Question 4. What is the best dosing strategy for immunosuppressives in Crohns disease, in terms of: starting and maximum doses, duration, dose escalation/deescalation (when? rate?), which immunosuppressive first?
Draft answer modified by National Meeting Working Group
(1) The most effective doses appear to be 2.0-3.0 mg/kg for azathioprine and 1.0-1.5 mg/kg for 6-mercaptopurine administered orally, based on reported clinical trials. There is no evidence to support dose de-escalation (level of evidence: 1a; grade of recommendation: A).
(2) For methotrexate, the dosing strategy should be 25 mg per week intramuscularly for 16 weeks as induction, and 15 mg per week in maintenance. There is no evidence to support further dose de-escalation (level of evidence: 1b; grade of recommendation: A).
(3) Azathioprine is recommended as the first-line immunosuppressive, as higher response rates were observed with it compared with 6-mercaptopurine and evidence for the use of methotrexate is scarce (level of evidence: 5; grade of recommendation: D).
(4) Recommended initial dose strategies are either a gradual dose increase starting with 50 mg of azathioprine (25 mg of 6-mercaptopurine) or full dose therapy (level of evidence: 1b; grade of recommendation: A).
(5) In case of full dose therapy, prior determination of thiopurine methyltransferase activity/genotype should be done whenever available (level of evidence: 5; grade of recommendation: D).
(6) Azathioprine or 6-mercaptopurine treatment should be maintained for several years due to a high relapse rate in patients with Crohns disease when these drugs are discontinued (level of evidence: 1b; grade of recommendation: A).
Question 5/Part 1. How should the efficacy of a treatment be monitored clinically and biologically? What is the definition of treatment failure? When should the effect of treatment be evaluated?
Draft answer modified by National Meeting Working Group
(1) Remission of signs and symptoms is the most widely clinically accepted endpoint for treatment efficacy. The Crohns Disease Activity Index and Harvey Bradshaw Index are accepted tools for quantification of efficacy in clinical trials, the latter is simple enough to allow its use in clinical practice (level of evidence: 5; grade of recommendation: D).
(2) Indirect biomarkers of treatment efficacy include:
(3) Elevated serum C-reactive protein (correlates well with disease relapse except for upper GI tract disease (level of evidence: 2b/4; grade of recommendation: B/C).
(4) Faecal calprotectin below the cut-off level of the individual test (predictive of mucosal healing and reduced relapse in Crohns disease) (level of evidence: 4; grade of recommendation: C).
(5) The use of azathioprine metabolites and trough infliximab or adalimumab levels may help management decisions and more accurately identify non-responders. For azathioprine users, metabolites are helpful in nonresponders. For biologics, trough levels cannot be recommended in clinical practice at this time (level of evidence: 5; grade of recommendation: D).
(6) For treatment with thiopurines or methotrexate, clinical response should be assessed after 3 months. However, if mucosal healing is to be assessed, this should be carried out at 6-12 months and at 2 years (level of evidence: 4/1b; grade of recommendation: C/A).
(7) For treatment with biological agents, clinical response should be assessed between 6 and 14 weeks (level of evidence: 1a; grade of recommendation: A).
(8) Patients failing to respond symptomatically after adequate therapy with thiopurines or methotrexate for at least 3-6 months or with a biologic for at least 6-14 weeks constitute a treatment failure (level of evidence: 2b - thiopurines; grade of recommendation: B).
(9) Considering immunossupressors and/or biologics, treatment failure should also include absence of endoscopic improvement (level of evidence: 1b --- biologics; grade of recommendation: A).
Question 5/Part 2. Should mucosal healing be assessed?
Draft answer modified by National Meeting Working Group
(1) Achievement of mucosal healing in Crohns disease leads to prolonged steroid-free remission, fewer abdominal surgeries and may reduce hospitalizations (Level of Evidence: 2b - remission; Grade of recommendation: B); (Level of Evidence: 4 - surgery; Grade of recommendation: C); (level of evidence: 2b - hospitalization; grade of recommendation: B).
(2) There is good evidence to suggest that azathioprine, infliximab and adalimumab are effective at healing the colonic mucosa completely (level of evidence: 2b; grade of recommendation: B).
(3) Early combined immunosuppressive therapy in moderately active Crohns disease is superior to standard therapy in establishing mucosal healing, mainly in naive patients for both drugs (level of evidence: 1b; grade of recommendation: A).
(4) Methotrexate and certolizumab are also capable of mucosal healing, although the evidence base is less firm (level of Evidence: 4 - methotrexate; grade of recommendation: C); (level of evidence: 3b - certolizumab; grade of recommendation: B);
(5) Although further studies are needed, monitoring of endoscopic response to biologics and/or immunossupressors should be recommended within 2 years (level of evidence: 1b/2b; grade of recommendation: A/B). Non-invasive markers such as C-reactive protein and in particular faecal calprotectin may become an alternative to endoscopy for the assessment of mucosal healing (level of evidence: 4; grade of recommendation: C).
Question 6. If azathioprine and a biologic are given in combination, should any of the treatments be stopped? Which treatment should be stopped to achieve the smallest reduction in efficacy? When should that treatment be stopped?
Draft answer modified by National Meeting Working Group
(1) In patients with moderately active Crohns disease naïve to immunosuppressive therapy, the combination of na immunosuppressive with infliximab improves rates of steroid-free remission up to 1 year after initiation of therapy (level of evidence: 1b; grade of recommendation: A).
(2) In patients refractory to immunosuppressive therapy, continuation of that therapy in conjunction with a biologic offers no clinical benefit up to 2 years (level of evidence: 1b; grade of recommendation: A).
(3) If the immunosuppressive is to be continued in conjunction with a biologic, then the immunosuppressive may be discontinued after 6 months. However, this decision must be individualized (level of evidence: 4; grade of recommendation: C).
(4) There is no evidence that immunossupressor and/or infliximab use are independent predictors for serious infections (level of evidence: 2b; grade of recommendation: B)
(5) Use of azathioprine is not associated with increased risk of cancer except for lymphoma (level of evidence: 3b; grade of recommendation: C).
(6) Up to now there is insufficient data to know whether use of biologics alone is associated with increased risk of lymphoma (level of evidence: 2; grade of recommendation: B).
(7) There is a small potential risk of hepatosplenic T-cell lymphoma in young males with Crohns disease being treated with a combination of azathioprine and infliximab (level of evidence: 4; grade of recommendation: C).
Question 7. If the immunosuppressive does not work, what should the approach be? Increase the dosage? Add steroids? Change the immunosuppressive? Move to a biologic?
Draft answer modified by National Meeting Working Group
(1) Optimization of thiopurine therapy should always be considered if underdosing is suspected on a dose/weight basis (level of evidence: 1a; grade of recommendation: A).
(2) Anti-TNF agents should be the first consideration in patients who did not respond to immunosuppressives or lost response (level of evidence: 1a; grade of recommendation: A).
(3) Adding steroids may be necessary in the short term, but they are not recommended for long-term use (patients should be weaned off steroids) (level of evidence: 4; grade of recommendation: C).
(4) In case of intolerance or side effects to azathioprine or 6-mercaptopurine, other immunosuppressives may be considered. Alternative immunosuppressives include: methotrexate (level of evidence: 1b/4; grade of recommendation: A/C) tacrolimus and 6-thioguanine (in limited settings only) (level of evidence: 4; grade of recommendation: C).
Question 8. If a patient experiences flare-ups when receiving immunosuppressives or a biologic, should corticosteroids be added?
Draft answer modified by National Meeting Working Group
(1) Patients failing immunosuppressive therapy can be started on corticosteroids to help induce remission when transitioning to another immunosuppressive (level of evidence: 1b; grade of recommendation: A).
(2) Biologics should be considered as both induction and maintenance agents, and transition with corticosteroids may not be necessary (level of evidence: 1b; grade of recommendation: A).
(3) When started, corticosteroid dose should be rapidly tapered over a period of weeks to avoid long-term exposure (level of evidence:3; grade of recommendation: B).
(4) Given their significant side-effect profile, use of corticosteroids should be limited or avoided where possible (level of evidence: 2b; grade of recommendation: B).
(5) If a patient loses response to a biologic, optimization of therapy should be considered (level of evidence: 1b; grade of recommendation: A).
Question 9. What are the risks of cancers (all kinds) and infections associated with the short-, mid- and long-term use of immunosuppressives and corticosteroids?
Draft answer modified by National Meeting Working Group
(1) Although the overall cancer risk does not seem to be increased in patients on steroids or immunosuppressives, thiopurines increase the risk of lymphoproliferative disorders and non-melanoma skin cancer in IBD patients (level of evidence: 2b; grade of recommendation: B).
(2) The concomitant use of immunosuppressive agents and biologics should be minimized, especially in adolescentes and young male adults (level of evidence: 4; grade of recommendation: C).
(3) Steroids and immunosuppressives are associated with na increased risk of infection (level of evidence: 2b; grade of recommendation: B).
(4) The risk of infection in patients with IBD increases with the association of corticosteroids and thiopurines and biologics (level of evidence: 3b; grade of recommendation: B).
(5) The concomitant use of corticosteroids and thiopurines or corticosteroids and biologics also increases the risk (level of evidence: 3b; grade of recommendation: B).
Question 10. What is the optimal safety monitoring (clinical, laboratory, radiological) of patients receiving immunosuppressives or corticosteroids? How often?
Draft answer modified by National Meeting Working Group
(1) Immunosuppressive therapy is associated with myelosuppression. Patients with low thiopurine methyltransferase (TPMT) activity are at increased risk of developing severe myelosuppression. However, 73% of patients with severe bone marrow suppression do not carry a TPMT mutation (level of evidence: 3b/5; grade of recommendation: B/D).
(2) As TPMT analysis may predict 90% of life-threatening episodes and 60% of severe and moderate episodes of neutropenia, measuring TPMT activity prior to starting thiopurines is a way to identify patients at high risk of severe haematological complications (level of evidence: 2a; grade of recommendation: B).
(3) It remains to be established whether measuring TPMT activity/genotype is a cost-effective strategy (level of evidence: 5; grade of recommendation: D).
(4) Patients receiving thiopurines need regular monitoring of full blood count before and within 4 weeks of starting therapy and then every 3 months with liver tests (level of evidence: 5; grade of recommendation: D).
(5) In patients receiving methotrexate, measurement of full blood count and liver function tests are advisable before and within 4 weeks of starting therapy, then monthly to every 3 months (level of evidence: 5; grade of recommendation: D).
(6) The EBV status of the patients submitted to immunosuppression should be assessed (level of evidence: 5; grade of recommendation: D).
(7) Nodular regenerative hyperplasia is a rare but potentially severe complication of azathioprine in patients with IBD (level of evidence: 5; grade of recommendation: D).
(8) Skin cancer screening programs should be performed according to a regular schedule (level of evidence: 5; grade of recommendation: D).
(9) Clinical monitoring of patients receiving doses of steroids is recommended, and doses should be tapered (level of evidence: not applicable).
(10) In patients with more than 3 months or recurrent courses of steroids DXA should be ordered (level of evidence: 5; grade of recommendation: D).
Conclusions
The main conclusions which can be drawn after this meeting include: the importance of introducing conventional corticosteroids in moderate to severely active Crohns disease of any localization with an initial duration of treatment varying according to patients response; in mildly active ileocecal and/or right-sided colonic disease the use of budesonide is recommended, this being preferred to conventional corticosteroids due to its safety profile. Furthermore, neither conventional steroids nor budesonide are effective for maintenance of remission.
Corticosteroids have been shown to increase the risk of serious and opportunistic infections, both independently and in combination with immunosuppressive and biologic agents. Thus, the best option to prevent steroidinduced side effects is to avoid prolonged or repetitive use and to switch appropriate patients to immunosuppressive therapy. Furthermore, the administration of immunosuppressives should be considered early in the disease course, particularly in patients at high risk of complicated disease.
For IBD the most important and, in clinical terms, most widely accepted endpoint for treatment efficacy is the remission of disease signs and symptoms. Therefore, all patients failing to respond symptomatically after adequate therapy with thiopurines or methotrexate for at least 3- months or with a biologic for at least 6-4 weeks are considered a treatment failure. With regards to immunossupressors and/or biologics, treatment failure should also include absence of endoscopic improvement.
The evidence that suggests that methotrexate is capable of mucosal healing is not as robust as the evidence supporting the effective and complete healing of the mucosa achieved with azathioprine, infliximab and adalimumab. Evidence also suggests that the early combination of immunosuppressive therapy in moderately active Crohns disease is superior to standard therapy in establishing mucosal healing, mainly in patients who are naïve to both drugs. The use of non-invasive markers such as C-reactive protein and in particular faecal calprotectin may become a complementary means to endoscopy for the assessment of mucosal healing.
Concerning the risk of cancer, there is evidence supporting an increased risk of developing lymphoproliferative disorders and non-elanoma skin cancer in IBD patients treated with azathioprine. Steroids and immunosuppressives are associated with an increased risk of infection. The combination treatment, immunomodulators and corticosteroids or biologics, increases this risk.
References
1. Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4(2):53-61. [ Links ]
2. Sartor RB, Sandborn WJ. Kirsners inflammatory bowel diseases. 6th ed. New York, NY: Saunders; 2004. [ Links ]
3. Loftus Jr EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17. [ Links ]
4. Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39(5):690-7. [ Links ]
5. Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53:1471-8. [ Links ]
6. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, et al. Long-term evolution of disease behavior of Crohns disease. Inflamm Bowel Dis. 2002;8:244-50. [ Links ]
7. Travis SP, Stange EF, Lemann M, Oresland T, Chowers Y, Forbes A, et al. European evidence based consensus on the diagnosis and management of Crohns disease: current management. Gut. 2006;55 Suppl. 1:i16-35. [ Links ]
8. Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009). (updated 2009 Mar; cited 2011 Aug 15). Available from: http://www.cebm.net/index.aspx?o=1025. [ Links ]
Conflicts of interest
The authors have no conflicts of interest to declare.
*Corresponding author
E-mail address: fm@med.up.pt (F. Magro).
Acknowledgements
The authors would like to thank to all the experts who participated and the remaining authors of the IBD ahead 2010 group (Dr. Paulo Caldeira, Hospital de Faro, EPE; Dr. Isabel Bastos, Unidade Hospitalar de Guimarães do Centro Hospitalar do Alto Ave, EPE; Dr. Luís Lobo, Hospital Pedro Hispano da Unidade Local de Saúde de Matosinhos, EPE; Dr. Paulo Fidalgo, Instituto Português de Oncologia de Lisboa Francisco Gentil, EPE; Dr. Leopoldo Matos, Centro Hospitalar de Lisboa Ocidental, EPE; Dr. António Marques, Hospital de Santa Maria do Centro Hospitalar de Lisboa Norte, EPE; Dr. Susana Lopes, Hospital de São João, EPE; Dr. Marta Salgado, Hospital Geral de Santo António do Centro Hospitalar do Porto, EPE; Dr. Fernanda Maçoas, Hospital Sousa Martins --- Guarda da Unidade Local de Saúde da Guarda, EPE; Dr. José Cotter, Unidade Hospitalar de Guimarães do Centro Hospitalar do Alto Ave, EPE; Dr. Susana Almeida, Hospital Pediátrico de Coimbra do Centro Hospitalar de Coimbra, EPE; Dr. Luís Lopes, Hospital de Santa Luzia de Viana do Castelo da Unidade Local de Saúde do Alto Minho, EPE; Dr. João Carvalho, Centro Hospitalar de Vila Nova de Gaia, EPE; Dr. Eugénia Cancela, Hospital de São Teotónio, EPE Viseu; Dr. Eunice Trindade, Hospital de São João, EPE; Dr. Luísa Barros, Hospital Padre Américo, Vale do Sousa do Centro Hospitalar Tâmega e Sousa, EPE; Dr. Raquel Gonçalves, Hospital de São Marcos, Braga; Dr. Rute Cerqueira, Hospital S. Sebastião do Centro Hospitalar de Entre Douro e Vouga, EPE; Dr. Paula Moura Santos, Hospital de Santa Maria do Centro Hospitalar de Lisboa Norte, EPE).
Disclosure: Abbott funded development of the publication by KeyPoint, Scientific Consultancy. Eva Leiria of KeyPoint, Scientific Consultancy provided medical writing and editorial support to the authors in the development of this publication. Abbott had the opportunity to review and comment on the publication content; however, all decisions regarding content were made by the authors.
Contributors: All the authors were involved with the whole process and maintained complete control over the direction and content of the paper.
Received 18 January 2012; accepted 19 January 2012













