Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.20 no.1 Lisboa jan. 2013
https://doi.org/10.1016/j.jpg.2012.04.013
The growing applicability of transluminal endotherapy in organized pancreatic necrotic collections
A crescente importância da endoterapia na resolução de colecções pancreáticas necróticas organizadas
Bruno Arrojaa,∗, Albano Rosab, Sandra Lopesb, Nuno Almeidab, Hermano Gouveiab, Carlos Sofiab
a Serviço de Gastrenterologia, Hospital de Santo André E.P.E., Leiria, Portugal
b Serviço de Gastrenterologia, Hospitais da Universidade de Coimbra, E.P.E., Portugal
*Corresponding author.
ABSTRACT
Acute necrotizing pancreatitis is a serious condition with multiple possible causes. In a proportion of patients it complicates with development of necrotic peripancreatic collections and in some cases these become infected. The latter is a strong indication for aggressive treatment, which has classically been laparotomy with open necrosectomy. Surgery has major possible complications yielding a field for alternative treatment options such as endoscopic drainage and more recently direct debridement through transluminal orifices. This report describes interventional endoscopy to treat two patients with large peripancreatic necrotic and infected collections, focusing on its advantages, limitations and future indications.
Keywords Endoscopic necrosectomy; Infected pancreatic necrosis; Endotherapy
RESUMO
A pancreatite aguda necrotizante é uma patologia severa com múltiplas etiologias, que pode em algumas circunstâncias evoluir com a formação de colecções peri-pancreáticas. A ocorrência de infecção nestas colecções é um evento sério e constitui uma indicação consensual para tratamento agressivo. A abordagem terapêutica clássica tem sido, ao longo de décadas, a laparotomia com necrosectomia que, porém, apresenta complicações dramáticas em muitos doentes. Por esta razão, têm surgido recentemente técnicas alternativas para resolução destas lesões como a drenagem endoscópica com desbridamento de material necrótico através de orifícios transluminais. Os autores descrevem a aplicação das técnicas endoscópicas no tratamento de duas doentes com colecções necróticas infectadas volumosas após pancreatite aguda necrotizante. São discutidas as suas vantagens, limitações e indicações futuras.
Palavras-Chave Necrosectomia endoscópica; Necrose pancreática infectada; Endoterapia
Introduction
The development of pancreatic collections may occur in diferente clinical set-ups. The most frequent causes are acute or chronic pancreatitis, neoplasms, surgery or trauma.1-3 In recent years, ERCP has become an important cause of acute pancreatitis as well, possibly leading to pancreatic collections in more severe cases.2,4
Pancreatic necrosis, which is defined as diffuse or focal areas of nonviable pancreatic parenchyma, develops in nearly 20% of patients and is accompanied with a mortality rate varying from 8 to 39%.5,6
Since 1992, peripancreatic fluid collections have been classified according to the Atlanta Criteria in order to decrease erroneous interpretations previously made.1,3,6 Additionally and for more practical purposes, pancreatic fluid collections may also be subdivided into three groups: (a) acute pancreatic-fluid collections; (b) pseudocysts; and (c) walled off pancreatic necrosis (WOPN). The latter was first used by Baron and his co-workers and refers to the contained sterile or infected mature necrosis which may develop several weeks after the acute inflammatory process.6,7
It is crucial to distinguish WOPN from the other mentioned fluid collections, and most importantly the presence of solid debris inside the collection since this is critical to determine the best therapeutic proposal.8
There are multiple ways of managing these collections, depending on their size, location, clinical symptoms and imaging findings.1,2,6,8 Accepted indications for drainage include chronic abdominal pain, upper GI obstruction (gastric or biliary), intolerance to oral feeding, significant weight loss and infection.1,2,6 Infected necrosis is virtually always na indication for intervention since it is the main determinant of multiple organ failure after necrotizing pancreatitis.1,4-9 Infection can be suspected or confirmed in the presence of fever, increased inflammatory serum parameters (such as leucocytosis or C-reactive protein), positive bacterial cultures of blood or fluid sample or presence of gas inside the collection on a CT scan.1,8
Necrotic collections drainage is amenable to distinct therapeutic modalities: surgery, endoscopy or percutaneous interventional radiology. Although surgery has been regarded as the most definitive and standard treatment procedure, it is also well recognized that it carries high mortality (6---39%) and considerable morbidity (19-69%) rates.5,8,10
For the past 15 years, in selected cases, endoscopic transluminal drainage with complete removal of infected necrotic tissue has been considered an alternative option to surgery. Results have been very promising and it has been consistently regarded to be as proficuous as surgery in controlling infection while being less invasive.1,4,6-8 This technique was pioneered by Baron and colleagues7 using stents and gastrocystic vigorous lavage through a nasocystic catheter. Few years later, Seifert9 first described an unprecedented direct retroperitoneal endoscopic necrosectomy, changing since then the course of endotherapy. This procedure may be accomplished by passing Roth-nets, snares, Dormia baskets or even the endoscope itself through the transmural entry site into the necrotic-containing cavity. These innovations set the path for the advent of natural orifice transluminal endoscopic surgery (NOTES).1,4-6,8-10 Resolution of necrotic infected collections improves with this strategy and has been reported to reach 81-93% with over 12-month follow-up periods.1,4,8
Case 1: A 30-year-old female was sent to our department after an episode of severe acute lithiasic pancreatitis three months earlier. Her current medication was oral pancreatic enzymes.
The patient had been complaining, for the previous weeks, of diffuse abdominal discomfort, occasional vomiting, progressive intolerance to oral feeding and weight loss. She had not noticed fever during this period. Laboratory data were as follows: haemoglobin 11.9 g/dL; leucocytes 4.6×103/_L, platelets 320×103/_L, INR 1.11, BUN 3 mg/dL, creatinine 0.57 mg/dL, alanine aminotransferase 11 U/L, aspartate aminotransferase 15 U/L, alkaline phosphatase 112 U/L, gamma-glutamyltransferase 24 U/L, total bilirubin 0.3 mg/dL, lactate dehydrogenase 161 U/L, serum amylase 320 U/L, C-reactive protein 10.3 mg/dL.
A contrast enhanced computed tomography (CECT) scan documented a large abdominal peripancreatic fluid collection with relatively well-demarcated borders, with 9 cm of greater diameter, inside of which semi-solid debris were seen (Fig. 1a ). The pancreatic duct appeared slightly dilated (4mm) in its distal segment. A magnetic resonance supported these findings.
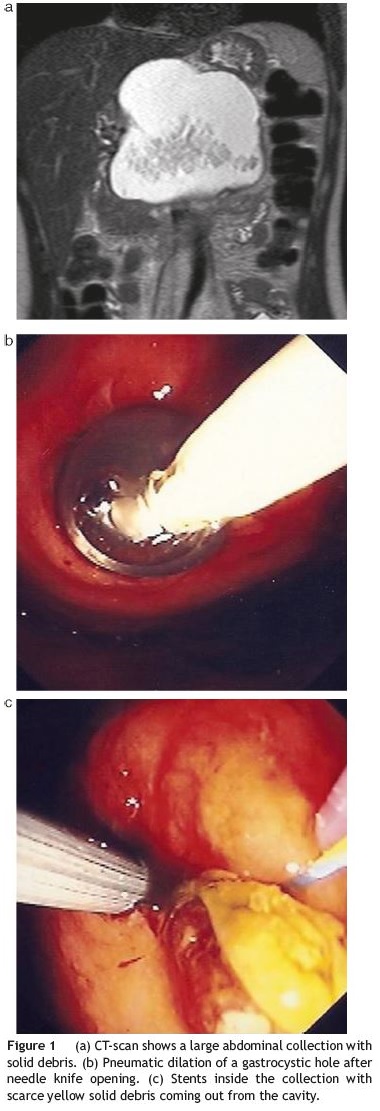
Percutaneous CT-guided drainage had been unsuccessful.
The patient agreed to undergo a transluminal endoscopic drainage of the peripancreatic collection under deep sedation. On endoscopy, a bulging lesion was evident on the greater curvature of the gastric body thus allowing direct opening with a pre-cut needle knife (Wilson-Cook Medical Inc.®) and introduction of a standard 0.035-in. Guidewire (Olympus®) followed by injection of contrast with opacification of the collection. Gastrocystic communication was dilated with a standard balloon (Olympus®) up to 10mm (Fig. 1b). A brown thick liquid with some solid yellow debris started to come out from the orifice. Three plastic 8.5F double-pigtail stents, 7-12 cm in length between flaps, and a nasocystic catheter were placed inside the collection (Fig. 1c). Subsequent saline lavage was done (2000 cc/24 h). An ERCP was performed on a second endoscopic session three days later, and despite no pancreatic duct leakage was seen, a decompressing sphincterotomy was done. The patient underwent three similar endoscopic sessions at days D8, D28 and D35 with pneumatic dilations of the gastrocystic orifice (maximal diameter 15mm) plus stent substitution until clear non-purulent fluid was seen draining out from the cavity. Follow-up CT-scans and fluoroscopy during endoscopic procedures confirmed the progressive shrinking of the collection until it completely disappeared. This was accompanied by excellent clinical and analytical response.
Case 2: A 48-year-old female developed a post-ERCP severe acute necrotizing pancreatitis. After initial management with conservative therapy during the first four weeks, she suffered clinical deterioration with fever, persistente epigastric abdominal pain, and intolerance to oral feeding with a palpable mass in the epigastrium. Laboratory data were also consistent with clinical worsening: leucocytes 28.7×103/_L, haemoglobin 10.1 g/dL, platelets 472×103/_L, INR 1.15, C-reactive protein 21.9 mg/dL, BUN 14 mg/dL, creatinine 0.75 mg/dL, albumin 3.2 g/dL, lactate dehydrogenase 154 U/L, alanine aminotransferase 10 U/L, aspartate aminotransferase 16 U/L, alkaline phosphatase 116 U/L, gamma-glutamyltransferase 99 U/L, total bilirubin 1.4 mg/dL, amylase 115 U/L.
A CECT-scan visualized peripancreatic fat densification and numerous communicating and confluent peripancreatic collections, extending inferiorly, with 11 cm×6.6 cm in size (Fig. 2a).
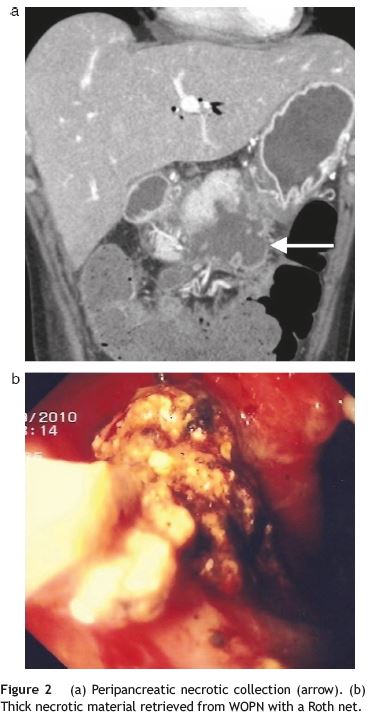
After patient consent, we decided to do a transluminal endoscopic drainage under anaesthetic sedation. A frank bulging on the lesser curvature of the gastric antrum enabled a direct gastrocystostomy with a pre-cut needle (Wilson-Cook Medical Inc.®) and placement of a standard 0.035-in. guidewire (Olympus®), after which balloon dilation (Olympus®) of the entry site to 15mm was done. The next step was access to the cavity with a Roth net (US Endoscopy®) which allowed extraction of large amount of solid brown necrotic debris (Fig. 2b). Three double-pigtail plastic stents, 7-8.5F, 7-12 cm in length between flaps, plus a nasocystic cateter for vigorous washing were inserted into the collection (2500 cc/24 h). A multi-resistant Escherichia coli was isolated from purulent material obtained for bacterial cultures.
We repeated three more endoscopic sessions at days D6, D15 and D35 since the first procedure. Since no further evidence of fluid drainage was seen during the last procedure, the stents were definitely removed and endoscopic treatment sessions were ended.
A CT-scan only detected a small liquid collection of 1.7 cm×2.9 cm, between the gastric antrum and the pancreas.
Laboratory data after last treatment was: leucocytes 6.2×103/_L, haemoglobin 11.4 g/dL, platelets 303×103/_L, C-reactive protein 1.29 mg/dL, albumin 3.9 g/dL, lactate dehydrogenase 160 U/L, alanine aminotransferase 29 U/L, aspartate aminotransferase 26 U/L, alkaline phosphatase 148 U/L, gammaglutamyltransferase 203 U/L, total bilirrubin 0.4 mg/dL, amylase 130 U/L.
Clinical outcome after follow-up was favourable. On the last appointment, the patient felt no pain, was tolerating normal oral feeding and had gained weight.
Discussion
It is of major importance to clearly establish the nature of a collection after acute necrotizing pancreatitis. A sterile asymptomatic necrotic collection can be managed conservatively.1,8 On the other hand, an infected or highly symptomatic peripancreatic necrotic collection merits a more aggressive approach because stopping the infectious process is crucial for the formation of granulation tissue.1-10 Classic management has been, for decades, open necrosectomy followed by postoperative drainage.2,5,9,10
The advent of new endoscopic techniques for the past twenty years, altogether with the considerable negative outcomes of open necrosectomy have been the main reasons why management of these serious complications has shifted. Percutaneous access was the first approach but, soon after, transluminal access with an endoscope started to take over with compelling results.2,4
Endoscopic drainage of necrotic peripancreatic collections has historically evolved from stents and nasobiliary catheters to the more recent direct retroperitoneal debridement.7,9 This happened because thick necrotic material may urge the need for additional transluminal necrosectomy, given that standard 7-10F stents may be insufficient for solid necrotic material elimination.1,2,4-9 Some authors initially argued that endoscopic ultrasound (EUS) should be used to assist draining procedures, but recent series do not report different outcomes in terms of efficiency or adverse events without the use of EUS given that a clearly visible gastric or duodenal bulge exists.1,2,6 We did not use EUS in our patients because an evident luminal compression was seen in both.
It is prudent to postpone endoscopic drainage and debridement for some weeks after onset of pancreatitis because this enhances a better demarcation of necrotic tissue from the viable pancreas, thus avoiding unnecessary risks.5,8 This was our attitude in both cases and it is unanimously supported from published experiences.4,6,7
We had no significant complications but multiple sessions were needed to definitively achieve complete evacuation of necrotic material. In the first case, there was not much solid material and therefore our strategy was to maintain stents and a nasobiliary catheter with intense saline lavage rather than doing necrosectomy. Conversely, the second patient had significant amount of thick solid material thus demanding aggressive debridement.
Limitations of endoscopic necrosectomy are the need for multiple sessions, endoscopic complications (e.g. perforation, bleeding, air embolism) and the lack of efficacy in large collections extending far away from the transluminal access point into the pelvis.1,4-6,8 Furthermore the experience of the endoscopist is of paramount importance.
Moreover, the lack of available specific endoscopic devices to retrieve necrotized material from a cavity is a relative restraint. Endoscopists have been improvising with ERCP and EUS equipment to overtake this problem.1 Manufacturers are expected to design novel tools which may possibly reduce the number of endoscopic sessions per patient whilst making the procedure simpler. An eventually useful tool might be a removable metallic stent placed in the gastro/enterocystostomy to allow easier drainage.1
Advantages of endoscopic intervention are considered to be its less invasiveness, fewer days of hospitalizations, faster recovery, less organ failure and secondary infections and better aesthetic outcomes.1,4,6,8 All these arguments are still certainly a matter of debate however, taking into account the lack of prospective randomized trials.
Considering our experience, we believe that a turning point in the management of peripancreatic infected and/or symptomatic necrotic collections has arrived. Endoscopic transluminal necrosectomy will probably expand as an alternative method to classic surgery. Nevertheless, this presumption is expected to occur in large tertiary hospitals since only these health-structures can more easily gather a multidisciplinary task force and high number of patients to bear large experience.
It seems reasonable to consider a step-up algorithm of treatment from conservative measures to endoscopic necrosectomy and ultimately surgery. Santvoort et al. sustain that by adopting this strategy, as much as 35% of patients can avoid surgery and total treatment costs decrease 12% for each patient.5 Selecting patients to one or another therapeutic technique has to be more clearly defined. Double-blind prospective randomized trials with homogenous patient population and long term follow-up are required, although we assume this will be very hard to achieve. This could help reducing selection bias from previous published series. It is reasonable to assume that worst patients more easily undergo laparotomy directly whilst less ill patients can be selected to undergo endotherapy firstly.1,4,5,8 As a consequence of this bias, mortality and morbidity outcomes are naturally expected to differ when we compare both options.
In conclusion, necrotic pancreatic collections are hard to manage and have an important impact on patients survival and health costs. New strategies have been being developed for alternative management including endotherapy, which is at the front line of investigation and practical applicability.
References
1. Voermans RP, Fockens P. Endoscopic treatment of pancreatic fluid collections in 2008 and beyond. Gastrointest Endosc. 2009;69:186-91. [ Links ]
2. Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635-43. [ Links ]
3. Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11-13. Arch Surg. 1993;128:586-90. [ Links ]
4. Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicenter study with long-term follow-up (the GEPARD Study). Gut. 2009;58:1260-6. [ Links ]
5. Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al., Dutch Pancreatitis Study Group. A stepup approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-502. [ Links ]
6. Coelho D, Ardengh JC, Eulálio JM, Manso JE, Mönkemüller K, Coelho JF. Management of infected and sterile pancreatic necrosis by programmed endoscopic necrosectomy. Dig Dis. 2008;26:364-9. [ Links ]
7. Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:820-3. [ Links ]
8. Voermans RP, Veldkamp MC, Rauws EA, Bruno MJ, Fockens P. Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos). Gastrointest Endosc. 2007;66:909-16. [ Links ]
9. Seifert H, Wehrmann T, Schmitt T, Zeuzem S, Caspary WF. Retroperitoneal endoscopic debridement for infected peripancreatic necrosis. Lancet. 2000;356:653-5. [ Links ]
10. Gardner TB, Chahal P, Papachristou GI, Vege SS, Petersen BT, Gostout CJ, et al. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085-994. [ Links ]
Conflicts of interest
The authors have no conflicts of interest to declare.
*Corresponding author.
E-mail address: brunoarroja@gmail.com (B. Arroja).
Received 14 January 2011; accepted 8 May 2011













