Introduction
1. Theoretical framework
The chestnut is one of the most useful trees in temperate regions with great social, ecological and economic value in mountainous regions, providing fruits of high carbohydrate food source and also timber. The chestnut fruit production is affected by the presence of lethal diseases as the Ink disease (Phytophthora cinnamomi Rands and P. cambivora (Petri) Buisman) and Chestnut blight (Cryphonectria parasitica (Murr.) Barr) as well as by insect pests (Cydia splendana HB, Curculio elephas Gyll and Dryocosmus kuriphilus Yasumatsu).
C. parasitica is a fungus of the OEPP list A2, responsible for the high current mortality of chestnut trees. It was first detected in 1904 on American chestnut (Castanea dentata (Marsh) Borkh) and was introduced in Italy in 1938 and progressively caused the destruction of large chestnut populations (Castanea sativa Mill.) in all European countries. Population biology and disease control of chestnut blight by hypovirulence have been presented in many and excellent reviews (Heiniger and Rigling 1994; Milgroom and Cortesi 1999; Robin and Heiniger 2001; Rigling and Prospero 2017). In Portugal, the disease has been present since 1989 (Abreu 1992) and is currently distributed throughout the country's chestnut regions. In terms of spatial distribution, the disease is characterized by the existence of places with a high incidence, with a high number of diseased trees, and other places with less extension and severity (Gouveia et al. 2001; Bragança et al. 2005). Characteristic symptoms of the disease are extensive necrosis (cankers) in the bark on branches and trunks that rapidly increase in size resulting in tree death (Rigling and Prospero 2017) causing severe environmental and economic costs.
This pathogen can be naturally infected by a hypovirus - Cryphonectria hypovirus 1 (CHV1) that induces modifications in morphological characteristics that include reduced asexual sporulation, and pigmentation capacity, it also diminishes the activity of pathogenesis-related enzymes, like oxaloacetate acethylhydrolase (OAH) (Chen et al. 2010) and laccase (Chung et al. 2008). Therefore, the introduction of hypovirulent strains has been used as a biological control method, which has been adopted and applied experimentally in Portugal since 2015 (Gouveia et al. 2016).
This study provides a new combination of biological and analytical approaches to evaluate the changes related to virulence morphological characteristics, oxidative enzymes activity and metabolic profiles of virulent C. parasitica and its isogenic hypovirulent after the infection of the fungi with the Cryphonectria hypovirus 1 (CHV1).
2. Methods
2.1 Isolates
C. parasitica isolates used in this study, listed in Table 1, were obtained from different chestnut field plantations in Trás-os-Montes region (Portugal). Isolates converted by the characterized hypovirulent strain RB111 (CHV1 donor) were also included in this study. These strains were maintained on Potato Dextrose Agar (PDA, 39g/L - Difco) agar slants at 6 - 8 °C.
2.2 Converted isolates
Three virulent isolates (Cast13, VBC02, Cast26) were converted with a characterized hypovirulent C. parasitica CHV1 isolate (RB111) by hyphal anastomose as described by Rigling et al. (1989). Mycelium of the converted strains was transferred to fresh PDA medium and maintained at 6-8 °C at the Instituto Politécnico de Bragança culture collection.
2.3 CHV1 detection
The presence of CHV1 (Cryphonectria hypovirus 1) in converted isolates was confirmed through molecular methods. Total RNA was isolated from mycelium using the NorGen BioTek kit (Thorold, ON, Canada). Extraction was done using lysis buffer, precipitated with 100 % ethanol and washed in columns with wash solution as manufacturer instructions. The RNA was dissolved in elution buffer and stored at -20°C. To obtain cDNA, 3μl of total RNA was diluted in 11μl of RNase free water and incubating at 100°C for 2 minutes. The RNA dilution was mixed with 4μl of 5x Reaction Mix (Sigma) and 2μl Maxima Enzyme Mix (Sigma) and incubated under the following conditions: 10 minutes at 25°C, 30 minutes at 50°C and 5 minutes at 85°C. The ORF-A region was amplified using the primers hvep-1F (5’- TGACACGGAAGCTGAGTGTC-3´) and EP-721-4 (5´-GGAAGTCGGACATGCCCTG-3´). For the ORF-B region, the primers orfB-12aF (5´-AGACCTCAATCGGGTCTCCCT - 3´) and orfB-12aR (5´- TTCAACCACACGACGAGTTCG - 3´) were utilized. PCR amplification was performed using 1 µl of cDNA in a total of 50 µl reaction volume consisted of 10 µl of 2X Jump Start (Sigma) and 1 µl of each primer (20 pmol/µl). Thermal cycling was set up with an initial denaturation at 94°C for 2 min, followed by 33 cycles consisting of 94°C denaturation for 1 min, annealing at 55°C for 1.5 min, and elongation at 72°C for 2 min, with a final extension at 72°C for 8 min. The PCR products were visualized by agarose gel electrophoresis on 1.5 % gel stained with GelRed® Nucleic Acid Gel Stain (Biotium, Inc) under UV illumination.
2.4 Virulence assays
2.4.1 Virulence test evaluation in apple fruits:
For this assay, five isolates of C. parasitica (Cast13, VBC02, RB111 (CHV1 donor), Cast13c, VBC02c) were used. A lot of homogeneous apples, without defects or decay, were washed in distilled water and dried. A sterilized Pasteur glass pipette was used to obtain circular mycelial sections of C. parasitica for inoculation in the apple’s holes made by a corky borer (5mm diameter). Each of the three replicates was identified and apples incubated at 24°C in the dark for ten days. The presence of brown lesions and their growth (length and depth) were evaluated in mm after ten days of incubation. The lesion area (cm2) was calculated from mean radii and the rot volume (cm3) was calculated by the mathematical formula of the cone.
2.4.2 Virulence evaluation in detached one-year growth branches of chestnuts:
Chestnut branches were collected in C. sativa nurseries at ESAB-IPB open fields. Chestnut branches were cut into sections with approximately 20 cm long. On both cut sides of the branches, paraffin wax was applied to avoid desiccation. In the middle of each branch a cork borer (3mm diameter) was used to excise a disk of the bark tissues, and inoculations of two virulent strains of C. parasitica (Cast13, VBC02) and three hypovirulent strains (Cast13c, VBC02c and the CHV1 donor RB111. The name of the isolate was registered in the branch. Then, the inoculation sites were covered with cotton wool moistened with distilled water and surrounded with parafilm to avoid desiccation. Finally, chestnut branches (three branches from each isolate) were placed on a tray and incubated at 25°C. Inoculated branches were checked for fungal growth after ten days. Fungal growth was assessed as the vertical and horizontal expansion of the visible necrotic area. The area of each necrosis was calculated using the mathematical formula for elliptic surfaces.
2.5 Qualitative enzymatic analysis
Nine strains listed in Table 1 (six virulent strains, represented by three isolates of vc type EU11 and three isolates of vc type EU66; and three (wild) hypovirulent isolates were used for qualitative evaluation of enzymatic activity in Petri plate assays. A set of tests was used to detect the activity of laccases, peroxidases (lignin peroxidases - LiP, and manganese peroxidases - MnP) and cellulases, enzymes associated with plant cell-wall degradation caused by fungi.
The capacity of C. parasitica strains to origin brown oxidation zones around the colony (in Bavendamm test), or to decolorize the dyes inside the media, creating a halo around the colonies (in the other tests) was evaluated in four different tests, with three replicates each.
Bavendamm test (phenol oxidase test): The medium contained 1.5% malt extract, 1.5% agar and 0.5% tannic acid, pH=4.5. The solution with tannic acid was prepared, autoclaved separately and mixed with the other components before pouring into Petri plates. These plates were inoculated with plugs from fungal strains and incubated at 25°C in darkness. This test was used to detect acid-tannic inducible laccases (as C. parasitica Lac3).
Screening for laccases and peroxidases (LiP and MnP): It was used malt extract agar (MEA) medium supplemented with 0.04% Remazol Brilliant Blue R (RBBR) and 200μM CuSO4. The solutions of 20% of RBBR and 400mM of CuSO4 were prepared and filtered through a 0.2μm filter. As a control, two uninoculated plates were used: one with the dye (as abiotic control) and one without dye (as biotic control).
Peroxidases test: For peroxidases evaluation it was used PDA with 25mg/l Azure B dye added. After sterilization it was aseptically transferred into rectangular Petri dishes, inoculated and incubated at 25°C in darkness.
Cellulase medium: The medium was prepared with 0.5% of carboxymethyl cellulose (CMC) and 1.6% agar for the growth of nine isolates. Four days after inoculation and incubation at 25°C, the plates were flooded with a 0.1% solution of Congo Red for 45min, then the stain was poured off, and they were destained with a 1M solution of NaCl for 15min. An uninoculated plate was used as a control for media discoloration.
2.6 Metabolic profile characterization
For metabolic profile characterization two virulent isolates (Cast13 and VBC02), their converted ones (Cast13c and VBC02c) and one hypovirulent strain (RB111) were grown in 250 ml Erlenmeyer with 100 ml of PDB (Potato Dextrose Broth, 24 g/L). Erlenmeyer’s were placed at 25°C in orbital shaking at 110 rpm for four days. After this the mycelium was filtered and washed with sterile water and disperser with an Ultra Turrax and transferred to glass bottles.
The global phenotypes and the utilization of 95 low molecular weight carbon sources (plus a negative control) by each of the isolates, were evaluated using the Biolog FF Microplate (Biolog Inc.), following manufacturer instructions. The obtained mycelium (viable and non-contaminated) was suspended in FF inoculating fluid supplied by Biolog in glass tubes (Cat. Nº 1006), mixed gently and adjusted to approx. 75 % transmittance at 590 nm using a Biolog Turbidimeter, previously calibrated using an FF Biolog Turbidity standard (Cat. Nº 3426). Mycelium suspensions (100 µl) was added to each well, and the FF MicroPlates were then incubated at 25°C in the dark. The optical density at 490 nm (mitochondrial activity) was determined using an ASYS UVM 340 microplate reader (Hitech GmbH) for each plate at 24 h intervals over the next seven days. Carbon sources were considered not utilized in wells in which colour development was less than, or equal to, that of negative controls. The capability of isolates to utilize different carbon sources was measured by average well-colour development (AWCD) (Garland and Mills, 1991).
2.7 Statistical analysis
Soft brown rot lesions caused by different isolates on apple fruit, and necrotic lesions on young detached chestnut branches were recorded and analysed using IBM-SPSS statistics, version 19 (SPSS Inc, 2010). Data of C. parasitica isolates were evaluated for normality with Kolmogorov-Smirnov and statistical analyses were performed using one-way analysis of variance (One-way ANOVA) followed by a post hoc test of LSD and Tuckey.
For the metabolic profiles analyses the average well colour development (AWCD) were calculated seven days after incubation, where AWCD equals the sum of the difference between the OD of the blank well (control) and substrate wells, divided by 95 (the number of substrate wells in the FF Microplate). AWDC of the five isolates, obtained seven days after inoculation, and the utilization of different groups of composts, were analysed using SPSS statistics analysis software, version 19 (SPSS Inc, 2010). Significant differences were determined by Duncan’s multiple range test, and P values less than 0.05 were considered significant. Multivariate analyses were performed in PAST v.3.18 to reduce the number of variables resulting from metabolic profiles (Biolog-carbon source utilization), using Principal Component Analysis (PCA).
3. Results
Conversion of virulent C. parasitica isolates and CHV1 detection
The results of conversions tests between each of the virulent strains: VBC02, Cast26 and Cast13 with the donor hypovirulent RB111 are shown in Figure 1A. Converted isolates (Cast13c, Cast26c and VBC02c) had no orange pigmentation and no sporulation when grown on PDA medium culture.
Hypovirulent strains (RB111, Cast13c and VBC02c), when compared with virulent ones showed a slow growth rate with an average of 6 mm per day, white mycelium and no spores production. On the other hand, virulent strains Cast 13 and VBC02 showed a higher growth rate with an average of 10 mm per day, orange mycelium colour, lobated colony margins and spores production.
Two converted strains were tested for the presence of hypovirus CHV1 and the donor strain RB111 was used as control. These results are shown in Figure 1B.
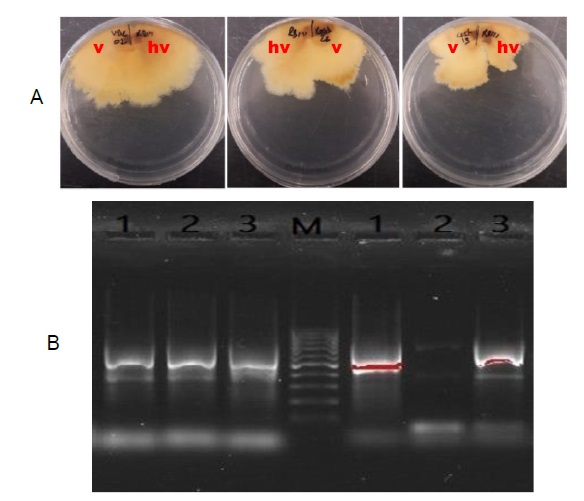
Figure 1 A: Conversion of virulent strains of Cryphonectria parasitica VBC02, Cast26 and Cast13 by RB111 the CHV1 donor. B: Detection of CHV1 hypovirus in converted strains of Cryphonectria parasitica using two different set of primers amplifying ORFA (right) and ORFB (left),(Lanes 1: Cast13c; 2: RB111; 3: VBC02c; M: 100 bp DNA marker).
Virulence evaluation tests
All inoculated apple fruits (cv. Golden Delicious) produced soft brown rot lesions (Figure 2B) and all inoculated detached chestnut branches developed necrotic tissues (Figure 3B). Apple fruit rot lesion measurements showed that rot tissue volume caused by VBC02 is higher than caused by its converted one (VBC02c). The pattern between Cast13 and Cast13c is not significantly different as shown in Figure 2A. Rot tissue volume of the hypovirulent RB111 is higher than Cast13, a virulent isolate.
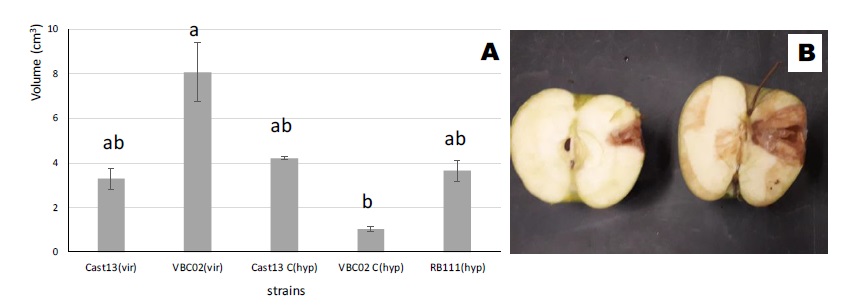
Figure 2 A: Mean brown rot volumes (mean ± SE) on apples (cv. Golden Delicious) caused by virulent and hypovirulent isogenic strains and the hypovirulent CHV1 donor of Cryphonectria parasitica, 10 days after inoculation of strains (Cast13, VBC02, cast13c, VBC02c, RB111). B - Necrosis on apples. Bars with the same letter(s) are not statistically different (p < 0.05). (vir - virulent, hyp - hypovirulent, for easier analysis of the graph).
Virulence evaluation by the detached chestnut branches lesions revealed the two virulent C. parasitica strains (Cast13 and VBC02) produced similar significant large lesions (Figure 3A). Hypovirulent converted isogenic strains (Cast13c, VBC02c) produced significantly smaller lesions than virulent isogenic strains. The donor CHV1 hypovirulent RB111 produced intermediate-sized lesions but not significantly different (p <0.05) from the virulent strain Cast13.

Figure 3 A: Mean necrotic lesion area (mean ± SE) on detached chestnut branches caused by virulent and hypovirulent isogenic strains and the hypovirulent donor CHV1 strain of Cryphonectria parasitica, 10 days after inoculation of strains (Cast13, VBC02, cast13c, VBC02c, RB111). B - Necrosis on branches. Bars with the same letter(s) are not statistically different (p < 0.05). (vir - virulent, hyp - hypovirulent, for easier analysis of the graph).
Qualitative enzymatic assays
The analysis of Figure 4 reveals that virulent strains (Cast13, Cast26, VBC02, Cast07, Cast17, VDP11) caused more intense brown oxidation zones and/or larger reaction areas in Bavendamm tests, concordant with higher Lac3 activity. Virulent strains also originated bigger discoloration halos than hypovirulent strains (RB111, Serra05, SR442) did, also indicating their higher production of other lignin-degrading enzymes (LiP and MnP) and cellulase. Between virulent strains, those from EU66 vc group (Cast07, Cast17, VDP11) showed higher cellulolytic activity when compared with those from EU11 vc group.
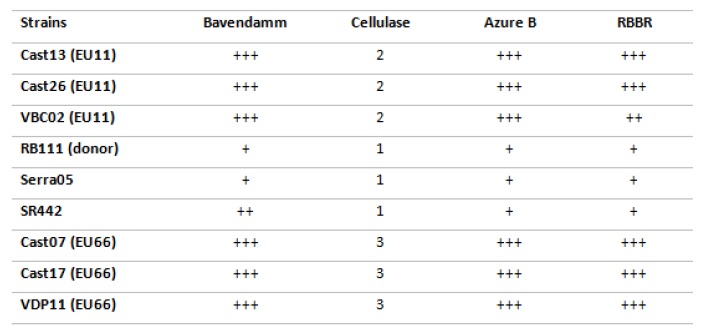
Figure 4 Results obtained in the screening tests used for the detection of enzymatic activity in Cryphonectria parasitica strains. Bavendamm test: + to +++ refer to increasing colour reaction/area obtained in the test; Cellulase test: 1 - refers to small ø halo around colony (0-5mm), 2 - medium size ø halo (5-14mm), and 3 - big ø halo (15-25mm); Dye discoloration in Azure B and RBBR media: + to +++ refer to increasing halo diameter.
Metabolic profile characterization
The Biolog FF MicroPlate, based on the company's Phenotype Array Technology, was recently introduced for the rapid identification and characterization of filamentous fungi. We used Biolog FF Microplates to characterize the metabolic profiles of virulent and hypovirulent C. parasitica isolates. These microplates contain 95 different carbon sources, and 75 of them were used by all the strains (Cast13, VBC02, Cast13c, VBC02c and RB111). None of the strains was able to use all types of substrates. The set of five strains studied were unable to utilize one carbon source: 2-amino ethanol. The virulent strain VBC02 consumed less carbon sources (85) than all the other strains, and the hypovirulent strain RB111 consumed the higher number of carbon sources (93).
Average well colour development (AWCD) of all carbon sources for the five C. parasitica strains is presented in Figure 5. After 7 days of incubation at 25°C, there were significant differences (p < 0.001), in metabolic capability among the five isolates, and the order was Cast13c > Cast13> VBC02c (Figure 5).
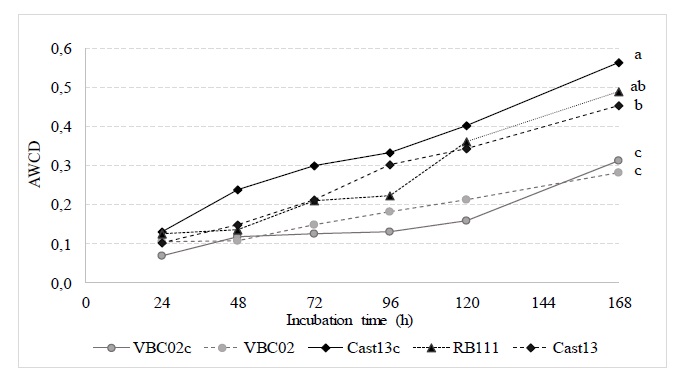
Figure 5 Average well colour development (AWCD) of all carbon sources for the five Cryphonectria parasitica strains (Cast13, Cast13c, VBC02, VBC02c and RB111). Mean (AWCD) of each isolate, after seven days of incubation at 25°C followed by the same letter are not significantly different (p <0.05).
According to the biochemical properties of carbon sources, the 95 substrates present in the FF Biolog Microplates (Biolog Inc.) were allocated in six chemical groups, including amines/amides, carboxylic acids, amino acids, carbohydrates, miscellaneous and polymers (Zhang et al. 2014). The results indicated that the utilization of six groups of carbon sources by the five C. parasitica isolates were significantly different (p < 0.001). The highest AWCD values were obtained for carbohydrates, carboxylic acids and polymers, and the lowest, for amines/amides, amino acids and miscellaneous (Figure 6).
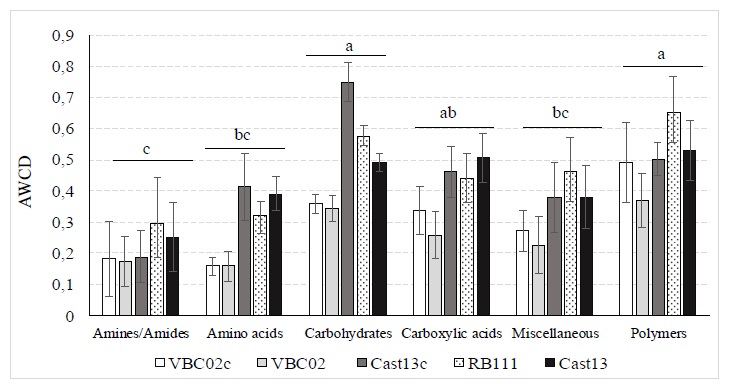
Figure 6 Carbon sources utilization by chemical groups, for five strains of Cryphonectria parasitica (Cast13, Cast13c, VBC02, VBC02c and RB111) after seven days of incubation at 25°C. Mean (AWCD) of each chemical group followed by the same letter are not significantly different (p <0.05).
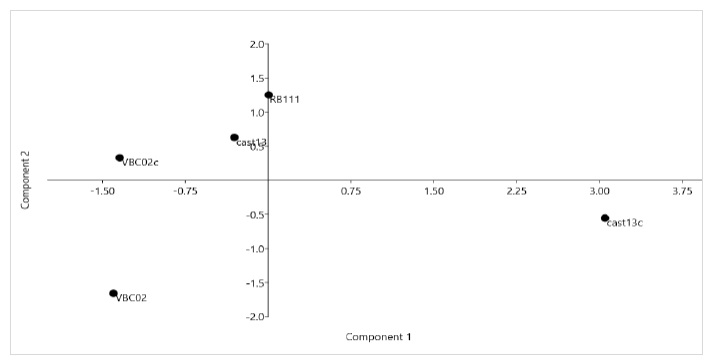
The PCA analysis shows that the strains studied have large differences in their metabolic profiles (Figure 7), especially Cast13c and Cast13 which are in different quadrants. The axis of principal component 1 (PC1) described 57.5% of total data variability and principal component 2 (PC2) described 22.4 % of total data variability.
4. Discussion
This work aims to compare virulent strains of C. parasitica and hypovirulent strains (which contain CHV1 hypovirus) applied as biological control agents. For virulence evaluation tests, apple fruits and detached chestnut twigs were largely used and considered useful for detecting marked deficiencies in virulence (Elisten 1985; Lee et al. 2006, Faruk et al. 2008). In this study, as a measure of virulence, we used the necrotic tissues developed by the inoculation of mycelia of each isolate in apple fruits (cv. Golden Delicious) or on detached branches of chestnut. All isolates grew under controlled conditions and the results on detached branches of chestnut prove to be more useful as an initial screening method to quickly detect a marked difference in virulence.
Tannic acid is abundant in the bark of chestnut trees and is considered to be one of the major barriers against C. parasitica infections (Chung et al. 2008). C. parasitica Lac3 is the enzyme that overcomes this host defence barrier, facilitating the fitness of the pathogen. In this work, nine different C. parasitica strains were screened on media containing substrates and indicator compounds that enabled the visual detection of ligninolytic enzymes and cellulase activities.
The more intense brown oxidation zones and/or larger reaction areas obtained with all virulent strains, (Cast 13, Cast26, VBC02, Cast07, Cast17, VDP11) in Bavendamm tests are concordant with a higher tannic-acid inducible Lac3 activity in these strains. These results are in agreement with Rigling et al. (1989) and Chung et al. (2008) that had previously reported that hypovirulent strains, infected with the CHV1 hypovirus dsRNA, exhibited a generalized decrease on laccases activity.
Virulent strains also originated bigger discoloration halos than hypovirulent strains (RB111, Serra05, SR442) did, also indicating their higher production of other lignin-degrading enzymes (LiP, MnP and cellulase). Between virulent strains, those from EU66 vc group (Cast07, Cast17, VDP11) showed higher cellulolytic activity when compared with those from EU11 vc group. Increased ligninolytic/cellulolytic activities seem to be concordant with higher strains virulence in C. parasitica, as it happens in other phytopathogenic fungi.
Five strains (two virulent, three hypovirulent - two converted and one original) were selected for further studies because they showed large differences in the qualitative assessment and they belong to the same VCG group (EU11), which resulted in a successful conversion. These strains were inoculated in different materials, namely apples and chestnut branches, to characterize their degree of virulence. Results obtained from the chestnut branches inoculation were related to the ones obtained previously with the indicators of ligninolytic enzymes production. Chestnut shoots are composed of lignin, hemicellulose and cellulose, therefore the lesions made by virulent strains, which have higher polyphenol oxidase (laccase, LiP peroxidase) and cellulase activities were more important than the damage made by strains that contain CHV1 hypovirus, which suffer from a decrease in laccase activity. Chung et al. (2008) have already referred that a laccase-null mutant caused a smaller lesion area on chestnut bark than did the virulent type. The reduction in the lesion area made by hypovirulent strains was also tested and proved in other studies, for C. parasitica (Chung et al. 2008) and in Botryosphaeria dothidea (Zhai et al. 2016) an infected fungus with a different type of hypovirus. Besides that, the biggest rot lesion was made by a virulent strain VBC02, and the smallest by a hypovirulent one - VBC02c. Different results were attained in the case of apple fruits inoculation in which a converted strain Cast13c caused a bigger infection area than its virulent version Cast13. This observation can be related to the reported results with the Biolog metabolic profile, that shows a high carbohydrates degradation by some hypovirulent strains, especially Cast13c. Carbohydrates represent the principal component of apples. Some of the hypovirulent strains tested showed great carbohydrates consumption but small rot lesion on apples. The reason for that can be due to the types of carbohydrates presents on apples and the different affinity of the enzymes secreted by C. parasitica strains for these substrates.
At the metabolic level, isolates were evaluated using Biolog FF MicroPlates, with concurrent reads of fungal utilization of 95 different carbon sources. Hypovirulent strains (Cast13c, RB111) showed great consumption of some substrates, and presented a very different metabolic profile comparing to their virulent version. Those hypovirulent strains are infected by the hypovirus CHV1, which is known to reduce the pathogenicity of the fungal but without completely cause mycelium dysfunction. On the contrary, the virus somehow looks like to keep the mycelium in a juvenile state while the debilitation is affecting pathways related to the virulence of the fungi. Another study also proved that the consumption of some amino acids, carbohydrates, lipids and nucleotides are increased in a virus-infected C. parasitica comparing to the wild type strain (Dawe et al. 2009). The fungal infection activates some metabolic pathways included in the fungal defence mechanism against the hypovirus. Some of these pathways require amino acids to activate antiviral mechanisms, they also consume different carbohydrates, which gives the mycelium the ability to take other pathways to produce energy or other compounds, and that explains the result from PCA analysis, which shows the big differences in the metabolic profiles between a virulent and its white strain.
Conclusions
In this work, all nine original strains were assayed for qualitative ligninolytic enzyme activity. At the same time, two virulent strains of C. parasitica were converted by hyphal anastomoses using a characterized hypovirulent strain. Then two virulent and three hypovirulent strains were chosen to test their virulence and metabolic profile. Results showed that the detached branches inoculation test is a suitable approach for the evaluation of marked virulence deficiencies. The necrotic lesion area developed by virulent strains is significantly larger than the caused by the isogenic hypovirulent strains. Besides, cellulase, laccase and lignin peroxidase activities are also reduced in hypovirulent strains. However, metabolic profiles evaluation assessed by Biolog FF microplates revealed that most of the carbon sources are more consumed by hypovirulent strains. The aforementioned achievements lead to the conclusion that the hypovirus does not cause a general debilitation of the fungus, but it partially modifies the genes related to the pathogenicity.
The metabolic analysis has revealed changes that happen in response to the hypovirus, it may also facilitate future isolate selection. As the use of specific carbon sources provides complete information on the isolates, those data can be put in a scientific database. These studies may lead to new perspectives for understanding the biological process used by the hypovirus.