Introduction
The European chestnut, Castanea sativa Miller (Fagacea) is a crop of great economic importance in some countries in southern Europe, mainly due to the value of the chestnut fruit. World production of chestnuts represented around 2.32 million tonnes (FAOSTAT, 2019) in 2017, of which 2.08 million tonnes were produced in Asia. The production of chestnuts in Europe represented around 152,000 tonnes in 2017 (FAOSTAT, 2019). Portugal is one of the most important European chestnut producers, with more than 34,000 tonnes produced in 2018, on about 38,000 ha of land (INE, 2019). In Portugal, there has been a significant increase in the chestnut area, especially in the region of the Trás-os-Montes, which represents about 85% of the national chestnut production area.
Chestnut quality is influenced by a wide range of biotic and abiotic factors. Of the biotic factors we can highlight attacks by pests and diseases. The commercial value of chestnuts is significantly harmed by pests or rot and decay diseases, leading to loss of income for farmers and for chestnut marketing and processing companies (Bento, Pereira & Pereira, 2007; Ruocco et al., 2016; Conedera, Thinner, Kreber, Rigo, & Caulldulo, 2016). The chestnut moth, Laspeyresia (= Cydia) splendana (Hübner), Cydia fagiglandana Zeller and Pammene fasciana L., pest complex and the weevil, Curculio (= Balaninus) elephants Gyllenhal, is identified as the most damaging in chestnut fruits in several European regions (Debouzie, Heizmann, Desouhant, & Menu, 1996; Speranza, 1999; Bento, Cabanas, Rodrigues, and Pereira, 2005; Avtzis, Perlerou & Diamandis, 2013; Conedera et al., 2016; Jósvai, Voigt, & Tóth, 2016; Beccaro, Alma, Bounous,& Gomes-Laranjo, 2019).
In the Trás-os-Montes region, of the pests mentioned above, the species C. splendana is the main one responsible for damage to chestnut fruit (Bento et al., 2007). The damage results from the formation of small galleries inside the fruit, as a result of the development of larvae inside. As a consequence, the fruits lose commercial value, and the incidence of attack may, under certain conditions, affect up to 50% of production (Bento et al., 2007).
The variety of chestnut, the presence of alternative hosts for the chestnut moth, the proximity of wild chestnut timber, the importance of parasitoids and predators, the climatic conditions and some cultural practices such as soil management, are some of the factors that can influence the population levels of C. splendana (Bento et al., 2007; Aguin-Pombo, Angeli, Aguiar, & Lopes, 2018).
Cydia splendana is a lepidopteran of the Tortricidade family, whose adult is a butterfly with a wingspan of 14 to 18 mm. During its life cycle, C. splendana goes through four stages of development (egg, larva, pupa and adult). Adults fly in the late afternoon, between early August and October. The females of the insect lay eggs, next to the midrib of the chestnut leaves, located close to the husks (Navarro, 2019). Laying begins 4 to 5 days after emergence and each female can lay up to 300 eggs (Aguin-Pombo et al., 2018). Two or three weeks after laying, the larvae hatch (Navarro, 2019), which go to the husks, opening holes in the chestnut fruit and penetrating inside. They then open galleries while they feed on the fruit (Aguin-Pombo et al., 2018; Navarro, 2019), taking between 35 and 45 days to develop. After this period, the larvae leave the fruit and bury themselves in the soil, where they spend part of their life cycle, in the form of a larva and later a pupa (Soares, 2008). In late July, early August, adults emerge (Bento et al., 2007; Aguin-Pombo et al., 2018). This species, like other species of moths, has only one generation per year (Aguin-Pombo et al., 2018; Bento et al., 2007; Navarro, 2019).
The chestnut moth is annually present in all Portuguese chestnut orchards, with high population levels (Soares, 2008). There are cultural practices that must be taken into account to minimize the attack of the pest such as: collecting the attacked fruits and later destroying them in order to kill the larvae that have not left them (Aguin-Pombo et al., 2018; Navarro, 2019) since these are likely to be the focus of infestation in the following year; carrying out shallow soil cultivations (10-15 cm deep) (Lopes et al., 2008). These cultivations, at the end of winter or in spring, expose larvae and pupae to the rigors of the climate, causing death and thereby reducing the population of the pest. Normally, cultivated chestnut groves have lower population levels of chestnut moth than non-cultivated chestnut groves (Soares, 2008; Aguin-Pombo et al., 2018).
A knowledge of the crop cycle of chestnut and its most susceptible phases, as well as an understanding of the biology of the pest, are essential for defining strategies for control, to minimize risks from biotic agents.
1. Methods
In six chestnut orchards located in the region of Trás-os-Montes, the insect was monitored at the different stages of development (immature states and adult) and the damage caused was recorded. The experimental fields for monitoring the pest are found in regions with different climatic conditions (DOP Terra Fria and DOP Padrela), located at different altitudes and with different cultural practices (Table 1).
Experimental chestnut orchards were subjected to different soil management practices. The chestnut orchards of Bragança (Espinhosela, Parâmio and Samil) and Vinhais (Espinhoso) were subject to superficial cultivations, once or twice a year. In the chestnut orchards of Macedo de Cavaleiros (Amendoeira) and Valpaços (Sobrado), they were covered with natural vegetation, destroyed two to three times a year (table 1).
1.1. Biology of the de C. splendana
To monitor the adults of C. splendana, in mid-June 2018 and 2019, delta traps with a sexual pheromone were installed in each of the experimental fields. In each orchard, three delta traps were installed, spaced at least 50 metres apart. The traps were suspended in the middle of the tree canopies (Figure 1A). Weekly, the traps were observed and the number of adults of C. splendana was recorded. The pheromone was replaced monthly and the glue reserves replenished whenever necessary. In four of the chestnut trees, a data logger was placed to record climatic data (figure 1B).

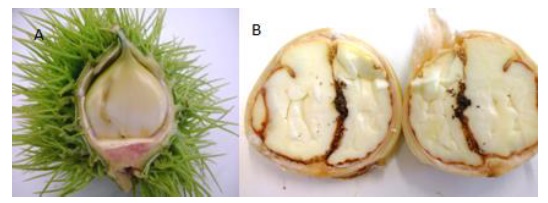
Figure 1 Delta trap with pheromone, placed inside the chestnut canopy, for monitoring of the chestnut moth, Cydia splendana Hübner (A), support with equipment for recording climate data - data logger (B), viable egg of Cydia splendana Hübner (C1), hatched (C2), predated (C3), fruit with hole of the chestnut moth larvae (D1), fruit dissected with the presence of chestnut moth larvae (D2).
The monitoring of C. splendana eggs began on 28 August in 2018, and one week later in 2019 (5 September), and continued weekly, for four weeks, in six chestnut orchards in 2018 and in five orchards in 2019. 200 leaves (10 leaves / tree for 20 trees) were collected, provided that they were located close to the husks, at different heights and positions within the tree canopy. The samples were taken to the laboratory in thermal boxes and were subsequently observed, using a binocular loupe, to account for the number of viable, hatched and predated eggs (figure 1- C1, C2 and C3).
After the flight peak of C. splendana, in two chestnut orchards (Espinhosela and Espinhoso), 25 husks were collected from four trees in each orchard. These were taken to the laboratory to open them and check for the presence of larvae of C. splendana, using a knife for dissection and a binocular loupe to find the larvae (Figure 2). Using this procedure it was possible to make a preliminary assessment of the damage caused by pests.
1.2. Damage caused by C. splendana
In order to assess the importance of the damage caused by the chestnut moth, in each of the six experimental chestnut orchards, three samplings of chestnut fruits were carried out during the 2018 harvesting period. In each sampling, 100 fruits were collected per orchard. The samples were taken to the laboratory, where they were dissected with a knife to look for larvae (Figure 1- D1 and D2). Additionally, the presence of weevil larvae or the incidence of fruits attacked by rot and decay diseases were recorded.
1.3. Data analysis
The data collected was stored in an Excel spreadsheet and later some descriptive graphs were produced, such as the C. splendana flight curve, taking into account the average number of catches per trap in each week and the standard error. For the evaluation of the damage, the intensity of attacked fruits was evaluated, taking into account the number of chestnuts infested or the incidence of disease in the different experimental orchards.
2. Results and discussion
In 2018, adult catches of chestnut moth were recorded between mid-August and early October, with the largest number of catches occurring in the last week of August in most chestnut orchards. In 2019, the flight period was shorter, with the last insect caught in the last week of September and the maximum number of adults caught in the traps in the second week of September, thus reaching a peak later than the previous year. This fact must be related to slight variations in the climatic conditions that were recorded in the two years of study. The flight period recorded in the present study was slightly shorter than that observed by Bento et al. (2009), in a study carried out between 2005 and 2008, which continued from mid-July to the end of October. In this same study, the maximum catch was observed in the middle of August (Bento et al., 2009), which does not coincide with what was observed in the present study. In Terceira Island (Azores), Lopes et al. (2008), also found a flight period between the end of July and the end of October.
In 2018, the Amendoeira orchard (Macedo de Cavaleiros), located at an altitude of 766 metres, recorded the highest number of insects captured, with 5.67 ± 0.88 (mean ± EP) per trap, at the peak of flight. In 2019, the highest number of catches was recorded in the Espinhosela orchard (Bragança), located at an altitude of 923 metres and with 10 ± 1.15 insects caught by the traps.
The smallest catches were recorded in Samil and Parâmio, with 0.33 ± 0.3 insects per trap, at the peak of flight (Figure 2) in 2018. In 2019, there were no catches in the Sobrado and Parâmio chestnut orchards.
The 2018 data shows that the altitude can influence the flight period, as well as the population levels of the insect, with the highest number of catches being recorded in the grove located at the lowest altitude (Amendoeira at 766 metres), with 1.67 ± 0.36 individuals per trap over the flight period. Henriques (2015) and Aguin-Pombo et al. (2018) also found that the highest incidences of chestnut moth attack occur in chestnut orchards located at lower altitudes, a fact that affects climatic conditions, and in particular, temperature. Lopes et al. (2008) also found that chestnut trees of lower altitude and those with a southern aspect were more frequently attacked by the pest. However, in 2019, this pattern was not observed, since the highest altitude (Table 1) recorded more catches, with 2.17 ± 0.42 insect per trap during the flight period (6 weeks). This situation is probably due to the climatic conditions that occurred during the adult emergency period during that year and the reduced number of cultivations carried out.
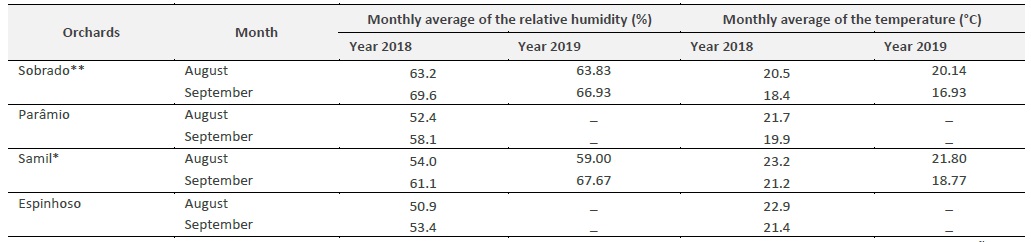
Table 2 Monthly average of the temperature and relative humidity, for August and September 2018 and 2019, in four chestnut orchards (Sobrado, Parâmio, Samil e Espinhoso).
Sources: in 2019 (*) Boletim Meteorológico para a agricultura do Instituto Português do Mar e Atmosfera (IPMA), (**) Sistema Nacional de Informação de Recursos Hídricos (SNIRH).
Climatic conditions, in particular temperature, precipitation, wind and humidity, affect the biology and behaviour of insects (Vásquez, 2011; Aguin-Pombo et al., 2018). In the present study, the lowest number of adults of C. splendana captured, was recorded in the chestnut orchards with the lowest average temperatures and highest relative humidity (average temperature in August was 20.5 ° C and the relative humidity 63.2%). This took place in 2018 in the Sobrado orchard, with an average of 1.6 ± 0.33 individuals per trap at the peak of flight (Table 2). On the other hand, when the relative humidity was lower (50.9%) and the average temperature was higher (22.9 ° C), the catches were higher as in the Espinhoso region, with 3.67 ± 0.58 insects caught on average, in 2018 (Figure 3). The same behaviour was seen for the adults of C. splendana in 2019 (Table 2, Figure 4).
With regard to soil management, the largest number of adult catches of C. splendana, over the flight period, was recorded in the orchard (Amendoeira) which had natural vegetation, destroyed two to three times a year (spring and autumn) ), with 1.67 ± 0.36 insects per trap. In the Samil orchard, where the soil was cultivated, fewer catches of adults of C. splendana were recorded during the flight period, with 0.17 ± 0.21 individuals per trap. According to Soares (2008), cultural factors assume great importance, providing a reliable means of protection, when it comes to soil management. Shallow tillage, exposing hibernating larvae and pupae to the rigors of climate, contributes to a lowering of the pest population (Soares, 2008; Lopes et al., 2008; Aguin-Pombo et al., 2018).
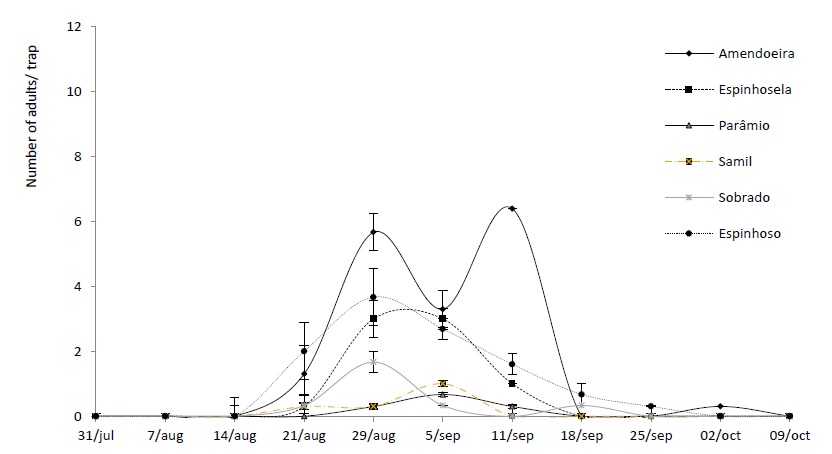
Figure 3 Average number and standard deviation of adults of Cydia splendana Hübner caught in a sexual pheromone trap, in the Trás-os-Montes region, in six experimental orchards in 2018.
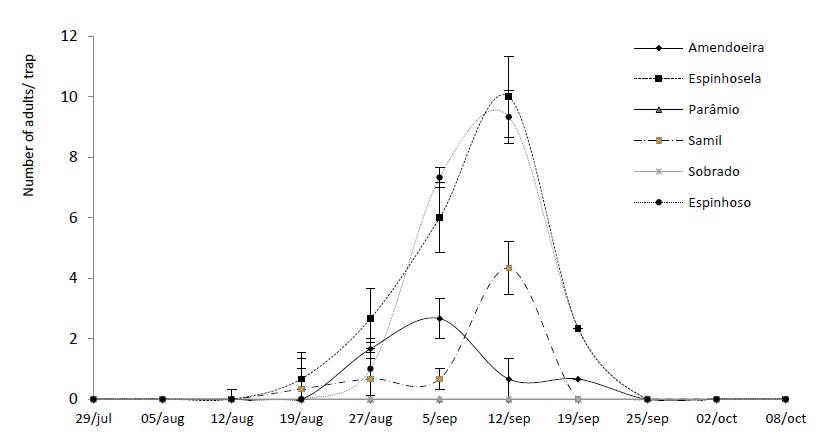
Figure 4 Average number and standard deviation of adults of Cydia splendana Hübner caught in a sexual pheromone trap, in the Trás-os-Montes region, in six experimental orchards in 2019.
With regard to immature states, the first viable eggs were observed one week after the first adult catches (last week of August) in 2018. The laying period was relatively short, with viable eggs being observed between late August and late September. At the beginning of September, the number of viable, hatched and predated eggs showed balanced values, but in the second week of September, hatched and predated eggs predominated. Hatched eggs thus define the beginning of the C. splendana larval phase (figure 5). In the same region where the present study was carried out, between 2005 and 2008, the first hatches of eggs of C. splendana were recorded in late July and the peak at the end of September (Bento et al., 2009). In 2019 it was only possible to register viable eggs in the first week of September, a week later (figure 6), with laying continuing until the end of September.
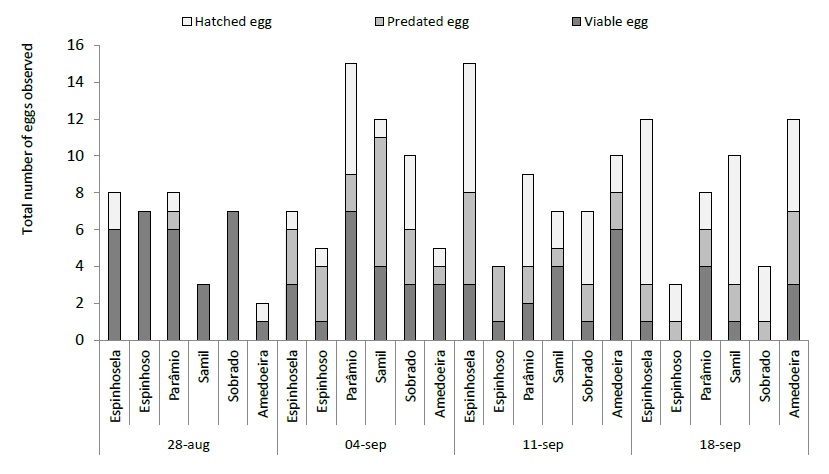
Figure 5 Total number of eggs of Cydia splendana Hübner (viable, hatched and predated) observed in six chestnut groves in the Trás-os-Montes region in 2018.
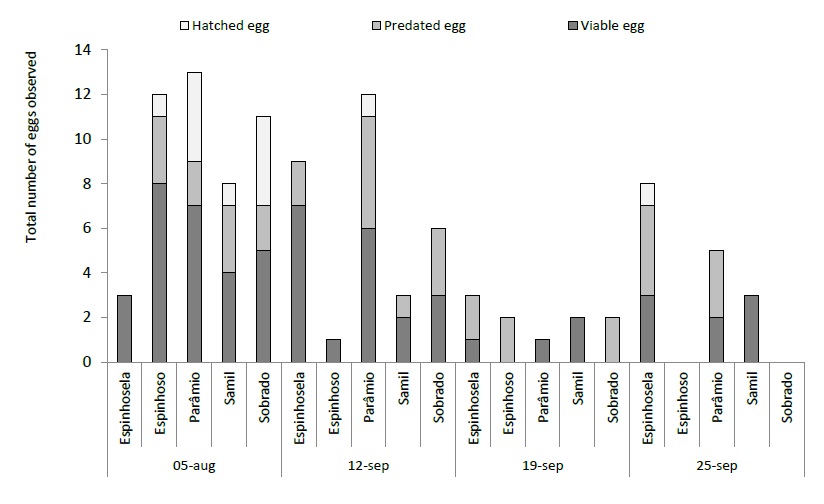
Figure 6 Total number of eggs of Cydia splendana Hübner (viable, hatched and predated) observed in six chestnut groves in the Trás-os-Montes region in 2019.
The preliminary assessment of the losses caused by pests shows that, on average, 42% of husks and about 20% of the observed chestnut fruits were infested.
As for the damage caused by pests and fungi, we observed average values of attacked chestnut fruit ranging from 13% in Sobrado to 35% in Espinhoso (Vinhais). Bento et al. (2007) recorded higher losses in 2004, in the same region, with values between 24% and 67.6%. In Espinhoso, 30% of the damage caused to the fruits was caused by pests, mainly by C. splendana (23%), followed by C. elephas (7%) (Figure 7). The results obtained in the present study are in agreement with those reported by Droga (2011), which indicates that about 25% of chestnuts delivered by the associates at the Cooperativa Agrícola de Penela da Beira presented chestnut moth and weevils. Henriques (2003) states that in cultivars most susceptible to chestnut moth attack, under favourable conditions, the losses can reach 80% of production. Also Romero (2013) and Cuesta et al. (2019) found that the attack of C. splendana, in Spain, depends on the cultivar in question among other factors, but consider C. splendana to be the main one responsible for damage caused in chestnut fruit. On the other hand, in Madeira, maximum attack values of C. splendana were found to affect as much as 40% of production (Lopes et al., 2008). In Greece, the losses attributed to C. splendana and C. elephas were 35.25% and 35.48%, respectively and these were considered the main chestnut pests in the country (Avtzis et al., 2013). Lower damage values were reported in Turkey where both species (C. splendana and C. elephas) were responsible for chestnut devaluation of between 15 and 20% (Karagoz, Gulcu, Hazir, & Kaya, 2009). In Italy, damage caused by pests reached 70.0% of production, with C. splendana and C. fagiglandana (Ebone et al., 2020) being principally responsible.
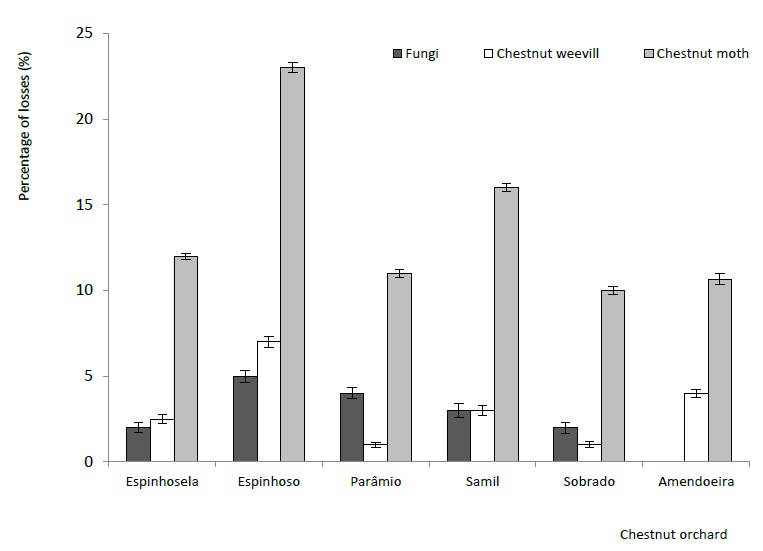
Figure 7 Importance of damage caused by pests (chestnut moth and weevil) and diseases (fungi), recorded in 2018.
It seems that the dominant chestnut pests can vary from region to region, within the same country. This depends on the biology of the pest, the existing hosts, climatic conditions and cultural practices. The existence of other hosts or chestnut trees and cultural practices seem to be the factors that most influence the population levels of C. splendana.
The incidence of chestnut fruits with fungi was found to be relatively low, on average, in the six chestnut orchards monitored. Only about 2.7% of the chestnut fruits had the presence of fungi, except in the Espinhoso region, where the presence of attacked chestnuts was higher (5%). On falling to the ground, the fruit is exposed to microbiological agents responsible for chestnut rot: Sclerotinia pseudotuberosa Rehm (Sinónimo: Ciboria batschiana Zopf., Sclerotinia batschiana; anamorfo de Rhacodiella castanea, sin. Myrioconium castanea) (Conedera, Jermini, Sassella, & Sieber, 2004; Donis-Gonzáles, 2008). According to Moscetti et al. (2014), the losses caused by fungi can reach up to 30% of the total harvest.
Conclusion
Catches of the chestnut moth were recorded between mid-August and early October, with the highest number of catches between late August and early September, in most of the monitored chestnut orchards. Catches were higher in chestnut orchards, which were located at lower altitudes and in chestnut orchards with the presence of an alternative host (chestnut timber) or with natural vegetation. Immediately after the peak of flight, it was possible to observe the presence of viable eggs. The hatched eggs indicated the presence of larvae of C. splendana, having been observed in the first week of September. The assessment of the damage caused by the chestnut moth varied between 10% and 23%, causing significant losses to producers and the agro-industry, so measures must be taken to minimize losses, thus safeguarding the commercial value of the chestnut.