Introduction
1. Theoretical framework
Initially detected in December 2019 in Wuhan, China, the new coronavirus (SARS-CoV-2) was pinpointed as the causative agent of the respiratory syndrome that in mid-2020 was named by the World Health Organization (WHO) COVID-19 (Coronavirus disease 2019) and decreed as a global pandemic (Mohamed & Alawna, 2020; Sharma et al., 2021). Currently, the disease has infected over 514 million people worldwide and has been fatal in 6.24 million cases. Studies that have analyzed hospitalization rates in several countries on different continents indicate that approximately 5% to 20.7% of those infected develop the most severe condition, requiring hospitalization for advanced care, emergency medical intervention with intensive care unit (ICU) care, and promotion of respiratory support (Wang et al., 2021; Yilma et al., 2021).
So far four main types of coronaviruses have been identified and are classified according to their propensity to infect certain cells and tissues and by their pathogenic power, they are: α, β, gamma, and delta. Three stages of increasing severity of infection by the new coronavirus are documented, ranging from asymptomatic states to its most severe form as a severe acute respiratory syndrome that may lead to hospitalization with the need for respiratory support or even multiple organ failure and death (Brugge et al., 2021; Polak et al., 2020). Thus, the treatment used for COVID-19 was initially based on the control and/or remission of the manifested symptoms, ranging from drug therapies, and transfusion to non-invasive and invasive ventilatory support therapies (Gavriatopoulou et al., 2021; González-Castro et al., 2021).
The use of non-invasive ventilation (NIV) as a form of ventilatory support when administered appropriately, with continuous monitoring, and with well-defined parameters promotes improvement in various respiratory parameters such as oxygenation and peripheral oxygen saturation, decreased respiratory work, and a significant reduction in the need for intubation and mortality (Navarra et al., 2020; Spadari & Gardenghi, 2020; Wang et al., 2021). The use of this therapy for patients with Covid-19 should always be seen primarily as an additional measure early in the disease process as part of a stepwise approach, at a time when the criteria for intubation have not yet been met or indicate that there is no need (Windisch et al., 2020).
The indication of NIV stems from the fact that it is widely used as a resource with significant results in patients with cardiopulmonary diseases such as Chronic Obstructive Pulmonary Disease (COPD) and Cardiogenic Pulmonary Edema (CPE). In such a way, viral pneumonia caused by the novel coronavirus (SARS-CoV-2), in its severe form (Severe Acute Respiratory Syndrome, SARS), produces severe hypoxemia refractory to oxygen therapy, with pathophysiological changes that have similarities to those found in Acute Respiratory Distress Syndrome (ARDS) and COPD exacerbation pictures. (Osadnik et al., 2017; Weng et al., 2010; Zhan et al., 2012).
Increased mortality as a consequence of delayed intubation and IMV may be linked to inadequate application of NIV and its form of monitoring. However, a clinical update for the treatment of patients with COVID-19 states that in settings with limited access to invasive ventilation or before patients develop severe hypoxemic respiratory failure, NIV may be a useful alternative (Hraiech et al., 2013; Murthy et al., 2020; Nava et al., 2011).
Given the global pandemic scenario caused by COVID-19, a disease that caused the search for hospital beds in many cases, strategies were adopted as the need for installation of NIV and even in more severe situations the IMV for reversal of SARS (Wendel-Garcia et al., 2022). These resources continue to be the subject of much investigation, thus the search for answers regarding the outcomes of NIV in this patient profile is justified as an important object of study. Thus, a systematic review will allow us to identify and analyze through scientific evidence whether NIV interferes in the hospital outcome of patients with the severe acute respiratory syndrome (SARS) triggered by COVID-19.
To this end, the following guiding questions were listed:
2. Methods
This systematic review protocol has already undergone registration submission and validation at PROSPERO under registration number: CRD42022337573.
The proposed systematic review will be conducted according to the JBI methodology for systematic reviews (Peters et al., 2020) and PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) recommendations (Moher et al., 2009).
Inclusion criteria
Participants
This review will include studies that elected hospitalized patients regardless of the ward, aged 18 years or older, who had a laboratory or radiological diagnosis of covid-19 and required noninvasive ventilation respiratory support in different modes and interfaces at some point during their hospitalization.
Intervention
This review will consider studies that evaluated the outcome of using NIV in different settings modes and parameters.
Comparison
The study will include articles that used other respiratory support techniques such as a high-flow nasal cannula, or oxygen therapy by a non-rebreather mask or nasal catheter as a comparator.
Outcome
Did the use of NIV change the length of hospital stay, interfere with the outcome of morbidity and mortality, or the rate of need for invasive mechanical ventilation?
Study type
This systematic review will consider literature published in peer-reviewed journals that cover all types of methodology, randomized clinical trials, experimental and quasi-experimental studies, and systematic reviews with meta-analysis, as well as studies published in the gray literature that meet the inclusion criteria for this study. Case studies, integrative reviews, protocols, guidelines, books, or letters to the reader will not be included.
Search strategy
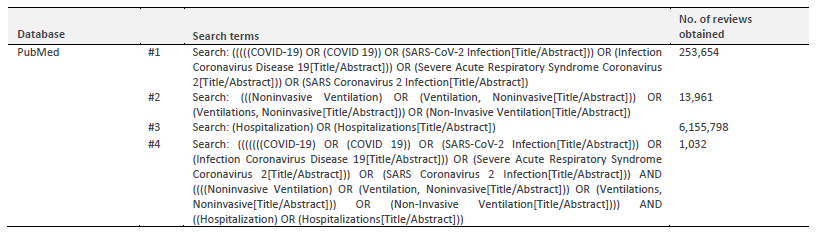
The search strategies used will delimit the studies that will be included in this review. In order to delineate the study, a first limited search was performed in the PUBMED database to find the scientific literature on the subject and use the Medical Subject Headings (MeSH), subject headings, keywords, and indexing terms of relevant articles on the subject to guide the next searches. In addition, the bibliographic references of articles analyzed during this initial stage of the review will also be used.
The following databases will be used for the study: PubMed, PEDro, Cochrane Central Register of Controlled Trials; Scielo, and grey literature in ProQuest Disserts and Theses Database Science; Web of Science, and Google Scholar as a purpose to obtain a comprehensive overview of the published literature. The proposed search strategy for the PubMed database, applied as an example, can be seen in Table 1.
Selection of studies and assessment of methodological quality
The selection of studies will occur through a screening procedure carried out by impartial reviewers, who will analyze the titles and abstracts of the scientific articles found in the research, after which a choice will be made of the full texts of the documents eventually selected for final insertion, taking into account the exclusion and inclusion criteria. Any disagreement about the choice or not of any study will be resolved through a third author, who will read the entire article and decide through argumentation and debate with the others.
The PRISMA flow chart will be used to display and specify the final report of all the results of the critical appraisal in narrative and/or tabular formats of each search step, including the reasons taken for the exclusion criteria. For analysis of study methodological quality, the two independent reviewers will use established assessment tools, where eligible studies will be critically assessed and characterized in their quality by the two reviewers. Each study that is included will go through the process of data extraction and summarization (when feasible), regardless of the assessment of the quality of its methodology.
To assess the reliability of the findings, The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) (Schünemann et al., 2013) will be used and a summary of the results will be created using GRADEPro GDT software (McMaster University, ON, Canada). The summary will contain the following information: absolute risks for treatment and control, relative risk estimates, heterogeneity, risk of bias, inconsistency, imprecision, and publication bias.
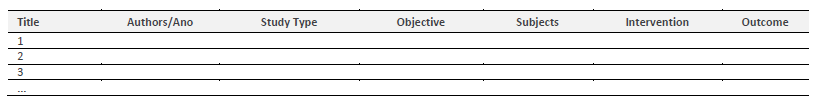
Data extraction, synthesis, and presentation
The data extraction will be done through an adaptation of the JBI data extraction tool (see table 2). This process will be done individually by each of the two evaluators and, after that, the other evaluators will review the homogeneity and authenticity of the extracted information impartially. The results of the study will be summarized through the use of tables to demonstrate the extracted data using organized and aggregated indicators to verify, present, and synthesize the evidence and findings related to the theme proposed for the research.
3. Results
The purpose of this systematic review is to include several references that point to the ways of administering NIV in patients with Covid-19-triggered SARS. An initial literature survey points out that the appropriate use of helmet-assisted NIV (HELMET) has significant outcomes on the rate of OTI and MV, but no significant difference in the number of days with support requirement (Grieco et al., 2021). Studies were also observed with outcomes on morbidity and mortality and protocols for use and monitoring (Duan et al., 2017; Guia et al., 2021), and forms of application and interfaces used (Sullivan et al., 2022).
4. Discussion
It becomes necessary to recognize whether the role and effect of the use of Non-Invasive Ventilation in patients with severe acute respiratory syndrome admitted for COVID-19, generated a positive impact or not on morbidity and mortality rates, prevention of orotracheal intubation, and discharges for improvement in the hospitals where these beings were admitted.
As well as to list the protocols used in various services, their forms of application, evaluation, and predictive indices of NIV failure, such as the HACOR scale and PaO2/FiO2 ratio (Duan et al., 2017; Guia et al., 2021). It is also necessary to recognize its indications and contraindications in this profile of patients, the safety practices related to the dissemination of contamination by COVID-19, and also the comparison with other techniques of ventilatory support/oxygen therapy (Grieco et al., 2021; Sullivan et al., 2022).
Since it is a disease with a worldwide impact and very high rates of hospitalization since the beginning of the pandemic, leading to reflection on the current reality of intensive care units in Brazil and worldwide. Thus, this study brings the need to identify what is being done in the current practice, what are the nuances and outcomes and use them as a basis for further inquiries.
Conclusion
The systematic review developed from the definitions and strategies presented in this protocol will help to identify pertinent questions about one of the main therapies used in hospital settings when it comes to SARS by COVID-19, which is the use of NIV as a way to avoid OTI to promote health based on scientific evidence and develop further knowledge on the subject.
It is also intended to inform the scientific, academic, and professional community of the current state of existing literature and identify possible gaps regarding the content studied, and thereby reference and/or study circumstantial suggestions to complement these existing gaps in the treatment of SARS by COVID-19. In addition, this study will inform the proper development and use of this positive pressure therapy to promote an appropriate and effective intervention in hospitalized patients.