Introduction
Pulmonary embolism (PE) occurs when there is a blockage of an artery in the lungs by a blood clot, which usually originates in the deep venous system of the lower extremities. They can also originate in the pelvic, renal, upper extremity veins or the right heart chamber (<10% cases) (Ouellette, 2020). Emboli can also originate from tumors, fat (long bone fractures), amniotic fluid, and foreign material during intravenous drug use. Clinical features are sudden onset of unexplained breathing difficulty, pleuritic chest pain, and hemoptysis. Signs include tachypnoea, tachycardia, hypoxemia, in severe cases, cardiovascular collapse and sudden death (Gibson, 2020).
Pulmonary embolism can occur after sustaining blunt chest trauma but is not a frequent phenomenon. Severe chest injury constitutes a newly recognized independent risk factor for pulmonary embolism in trauma patients (Jancin, 2011). Pulmonary embolism is usually a complication that, on average, occurs 4-7 days after serious injury (Manekar, 2007). The majority of pulmonary embolisms occurred during the first week of hospitalization, with some as early as 24 hours and none later than 15 days post-trauma (O`Malley, 1990). However, there is no definitive explanation for the cause and timing of pulmonary embolism in trauma patients (Lewis, 2013). Tissue injury after trauma can contribute to endothelial damage, which in turn can lead to hypercoagulability (Marx, 2007). We report a case of a young male patient who was diagnosed with pulmonary embolism after four days of blunt chest trauma.
Literature review
(Siddiqui et al, 2022) Siddiqui et al. mentioned that 33% of the reported pulmonary embolism (PE) incidents are directly related to early pulmonary embolism. The researchers used a retrospective design in this study and suggested a prospective one. The exact information of PE in trauma cases is generally independent of deep vein thrombosis (DVT), as described in this paper, and the risk of dreaded problems can be addressed by in vivo and in vitro studies. Higher rates of PE in trauma patients can be minimized by avoiding the delay in chemoprophylaxis.
1. Methods
Case presentation
A 30-year-old male patient presented to the emergency department with an accidental history of a fall in the bathroom four days back. The patient had a blunt injury to the chest. He developed breathing difficulty associated with fever, chest pain, and expectoration four days after the fall, for which he came to the hospital for evaluation and management. There was no significant past medical & drug history, no history of immobilization or malignancy.
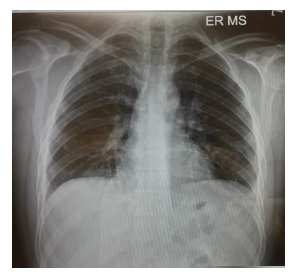
On initial examination in ED (Figure 1), pulse rate (PR) was 110/min, BP- 120/70 mmHg & Peripheral Oxygen Saturation (SPO2) was 89% on room air. An electrocardiogram (ECG) showed sinus tachycardia, and the Chest X-ray was normal. Figure 1 clearly indicates a small wedge-like triangular density in the left lower lobe of the right lung (Humpton’s hump).
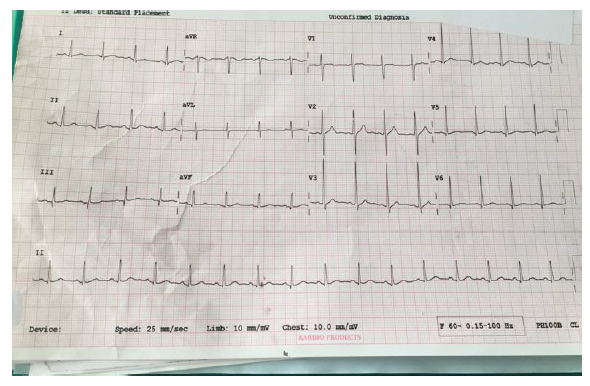
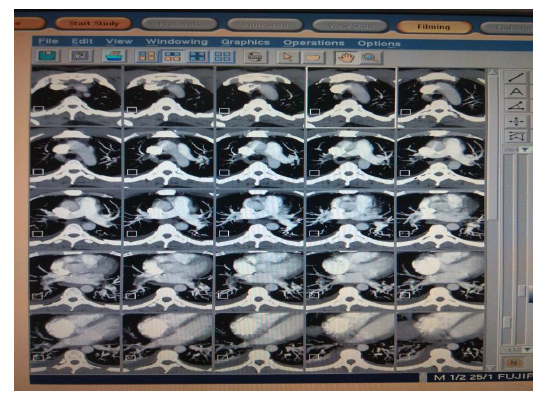
The patient was admitted to the ward and treated with IV antibiotics, analgesics, antipyretics, and oxygen therapy. Computed Tomography (CT) Chest (Figure 2) showed peripheral patchy areas of consolidation/atelectasis-like changes in the right middle, right lower lobes, and left lower lobe. It also shows the patient's lead Electrocardiogram (ECG), showing right ventricular strain (S1Q3T3 pattern), with mild T wave inversions in anterior leads.
But in spite of treatment, his symptoms started to worsen; he had tachypnea, persistent hypoxemia, and chest pain. RRT was announced in the ward, and the patient was shifted to the Medical Intensive Care Unit (MICU) for further care and stabilization. In MICU, the patient was kept on NIV for hypoxemia, and analgesics were started. 2D ECHO was done, which showed mild MR (Mitral Regurgitation), mild TR (Tricuspid Regurgitation), and EF (Ejection Fraction) approx. 55%, RVSP 55mm Hg (Right Ventricular Systolic Pressure).
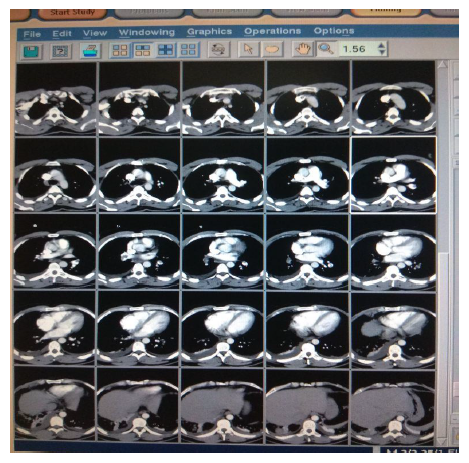
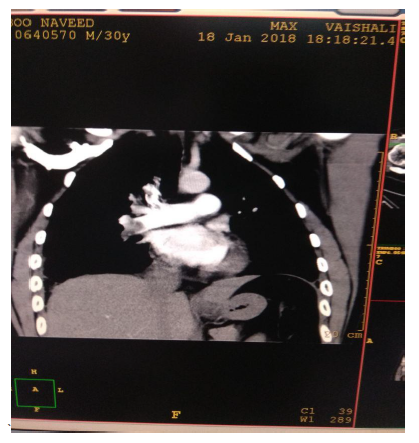
In view of high suspicion of pulmonary embolism in ECHO, CT Pulmonary angiography was done (Figure 3) which showed moderate pulmonary thromboembolism with evidence of hypodense thrombus seen at ramification of bilateral pulmonary arteries (Rt>Lt) extending in segmental branches causing marked occlusion of segmental branches of right lower lobe & lateral segmental branch of left lower lobe & partial occlusion of segmental branches on bilateral upper & lower lobes. A bilateral lower limb Doppler (Figure 4) was done, and no evidence of DVT was found. Treatment with LMWH was initiated. Intravenous thrombolysis was not planned as the patient was hemodynamically stable. A Thrombophilia profile test was sent, which came to be negative. Trop I was 0.06 & NT -proBNP was 426. He responded initially to conservative management, but however, he had persistent high O2 requirement and tachypnea requiring NIV support. He continued to have chest pain on day 5 of ICU admission. His repeat echo was done, which showed dilated RA (Right Atrium) and RV (Right Ventricular) with RVSP 65 mmHg. In view of severe symptoms and right ventricular dysfunction, the patient was planned for catheter-directed thrombolysis. CT Pulmonary Angiography of the Patient showed hypodense thrombus at the ramification of bilateral pulmonary arteries (Rt>Lt) extending in segmental branches of both Right and left lower lobes.
Catheter-directed intra-arterial thrombolysis was done (Figure 5) with Reteplase, and two bolus injections of 5 units at an interval of 20 minutes were given by an interventional radiologist.
The patient was then started on Inj. Heparin infusion for clot dissolution and stabilization with APTT monitoring. The next day, ECHO was done, which showed improvement in RV function, normal cardiac chambers, trace MR, TR, RVSP 45 mmHg. He remained hemodynamically stable, his oxygen requirement reduced gradually, and he shifted out of the ICU. He was then discharged and started on a novel oral anticoagulant, i.e., Rivaroxaban therapy.
2. Results
Our patient was discharged on the novel oral anticoagulant Rivaroxaban. It is one of the first available direct factor Xa inhibitors, recommended for use in venous thromboembolism. It allows predictable anticoagulation and routine coagulation monitoring is not required, unlike warfarin (Electronic Medicines Compendium, 2023).
3. Discussion
Acute pulmonary embolism in young adults is not very common. Pulmonary embolism can present without DVT i.e., can originate de novo (DNPE). In one series, i.e., 11,330 patients evaluated by trauma service, DNPE seems to be more prevalent after trauma in 61%, likely representing a local response to injury or inflammation; however, further research is warranted (Van Gent et al., 2014). There is still no definitive explanation for the cause and timing of pulmonary embolism in trauma patients. Coagulation and fibrinolysis are triggered immediately after trauma due to direct tissue injury. The incidence of pulmonary embolism was shown to be 0.5% in high-risk patients (multiple fractures or spinal cord injuries) and 0.2% in patients not at increased risk. Our patient Well’s score (1.5) and pulmonary embolism severity index score (<65%), both were low risk for pulmonary embolism (Van Gent et al., 2014). Our case was not thrombosed systemically as the patient was hemodynamically stable; therefore started with a therapeutic dose of LMWH. The treatment of choice for small to submassive pulmonary embolisms with hemodynamic stability is standard anticoagulation like low molecular weight heparin, unfractionated heparin, factor Xa inhibitors, and direct thrombin antagonists (U Nazir, 2022). However, due to worsening symptoms, RV dysfunction, and hemoptysis, the patient was taken for catheter-directed thrombolysis. About 40% of patients with acute pulmonary embolism have normal blood pressure but can have some evidence of RV dysfunction (Sharma et al., 2022). Assessment of RV dysfunction by ECHO is helpful in guiding aggressive treatment like thrombolysis (ten Wolde et al., 2004). Catheter-directed thrombolysis with Reteplase is rapid and safe in comparison to systemic thrombolytics as the dose requirement is reduced and has less risk of hemorrhage (Kuo et al., 2009). Reteplase is a plasminogen activator used in the treatment of occlusive thrombotic disorders (Simpson et al., 2006).
Conclusion
It is important for clinicians to consider pulmonary embolism in the differential diagnosis of patients with respiratory compromise, hypoxia, and chest pain, especially in thoracic trauma other than atelectasis, hemothorax, lung contusion, or pneumonia (Marx J. A., 2007). Trauma produces a state of hypercoagulability (Selby, 2009), which can result in pulmonary embolism. Pulmonary embolism remains a disease that requires clinical suspicion so as not to miss the fatal disease. Regional thrombolysis is safe and effective in certain conditions over systemic therapy. The results obtained from this analysis conducted retrospectively are anticipated to contribute to raising awareness of early diagnosis of embolism in the context of trauma. The practitioner follows the defined treatment protocol.
Abbreviations
APTT: Activated Partial Thromboplastin Time, ECG: Electrocardiogram, ED: Emergency department, CT: Computed Tomography, EF: Ejection Fraction, RRT: Rapid response team, NIV: Non-invasive ventilation, DVT: Deep venous thrombosis, LMWH: Low molecular weight heparin, n T pro BNP: N terminal pro b-type natriuretic peptide, PE: Pulmonary embolism, RVSP: right ventricular systolic pressure, MR: Mitral Regurgitation, PR: Pulse Rate, SPO2: Peripheral Oxygen Saturation, TR: Tricuspid Regurgitation, RA: Right Atrium, RV: Right Ventricular.
Author contributions
Conceptualization, S.G. and H.G.; data curation, S.G. and H.G.; formal analysis, S.G. and H.G.; investigation, S.G. and H.G.; methodology, S.G. and H.G.; project administration, S.G. and H.G.; supervision, S.G. and H.G.; validation, S.G. and H.G.; visualization, S.G. and H.G.; writing-original draft, S.G. and H.G.; writing-review and editing, S.G. and H.G.