Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Psicologia, Saúde & Doenças
versão impressa ISSN 1645-0086
Psic., Saúde & Doenças vol.18 no.2 Lisboa ago. 2017
https://doi.org/10.15309/17psd180214
Inteligência e diabetes mellitus do tipo 1
Intelligence and diabetes mellitus type 1
Nietsnie Duarte1,2, Izabel Hazin,1,3,*,Carolina Vilar1,4, Amanda Guerra1,5, Luana Metta1,6, Danielle Garcia1,7, Rosália Freire1,8
1Universidade Federal do Rio Grande do Norte – UFRN, Natal, CEP 59066-800 – RN, Brazil.
2e-mail: nietsnieduarte@gmail.com.
3e-mail: izabel.hazin@gmail.com.
4e-mail: carolinabvilar@gmail.com.
5e-mail: adlbguerra@hotmail.com.
6e-mail: luanametta@hotmail.com.
7e-mail: daniellefg@yahoo.com.br
8e-mail: rosaliacarmen@yahoo.com.br
Endereço para Correspondência
RESUMO
O objetivo deste estudo foi avaliar a habilidade intelectual de crianças com Diabetes Mellitus do Tipo 1 (DM1). Para tanto, foram consideradas as variáveis clínicas: idade ao diagnóstico e exposição a episódios de hipoglicemia. Utilizou-se como medida de avaliação da inteligência o WISC III. 20 crianças com DM1, de ambos os sexos e com idades entre seis a nove anos, participaram do estudo. Os dados obtidos foram interpretados a partir de análises descritivas e inferenciais. Observou-se que 80% dos participantes obtiveram rendimento global classificado dentro da variação normal de inteligência. As pontuações mais baixas obtidas pelas crianças diagnosticadas com DM1 foram identificadas no dominio da Organização Perceptual. A análise do impacto das variáveis clínicas sobre o desenvolvimento da inteligência apontou diferenças significativas entre crianças com início precoce da doença quando comparadas àquelas com início tardío, nos diferentes subtestes que compõem a escala. Os dados sugerem que a DM1 está diretamente relacionada a alterações no funcionamento cognitivo, especialmente em casos de início precoce da doença.
Palavras-chave: diabetes mellitus tipo 1, inteligencia, desenvolvimento infantil.
ABSTRACT
The aim of this study is to evaluate the intellectual capacity of children with DM1. We considered the clinical variables: onset of disease and exposure to episodes of severe hypoglycemia. The WISC-III was used for evaluating the intellectual abilities of the participants. 20 children with DM1, of both sexes and aged six to nine years, participated in the study. We carried out the descriptive and inferential analysis of the data. We observed that 80% of the participants had a global performance classified within the normal intelligence range. Considering the four Index Scores, we observed that the children with DM1 obtained their lowest scores on the Perceptual Organization Index. Regarding the possible influence of clinical variables, this study found significant statistical differences between children with early onset of DM1 and late onset in subtests of the WISC-III. These data suggest that DM1 is causally related to alterations in cognitive functioning, especially in cases of early beginning of the disease.
Key-words: diabetes mellitus type 1, intelligence, child development.
Diabetes mellitus type 1 (DM1) is considered a polygenic inheritance disease modulated by environmental factors. It covers about 5-10 percent of all cases of DM and has its onset typically in childhood or adolescence, with bimodal distribution. The first peak happens between 4-6 years old, and the second between 10 and 14 years. This is the most common type of diabetes in those stages of development (Gan, Albanese-O'Neill & Haller, 2012). The incidence of DM1 is increasing in many countries. It varies according to region, but the annual general growth has been marked at approximately 3 percent. In this sense, the importance of the disease as a public health problem is already internationally recognized (Patterson et al., 2013).
In Brazil, it is estimated that approximately 400 to 800 thousand people suffer from DM1 (Alves, Veiga, Toralles & Ribeiro, 2014). The country presents strong inequality between regions regarding access to treatment and monitoring of diabetic patients. The North and Northeast regions, which have the worst human development indices, showed, between 2006 and 2010, the highest mortality rate from diabetes in the country (Klafke et al., 2014). These data suggest difficulties in accessing health services, including emergency care, insulin delivery and attention to acute infections.
Those conditions are important to consider in the clinical picture, since the glycemic control is not ideal, which is a problem. The frequency and intensity of episodes of hypoglycemia and hyperglycemia and the lack of control results in problems in the dosage of insulin, diet control, and this situation interferes with child development especially in neurodevelopment. That occur because the human brain needs a certain amount of glucose to perform its functions optimally. If a fall of blood glucose occurs suddenly, the four counter-regulatory hyperglycemic hormones (cortisol, adrenaline, glucagon and growth hormone) are mobilized to reverse the situation. However, in certain circumstances, such as long duration, or excessive insulin dosage and poor control, the action of these hormones is not enough to avoid hypoglycemia (Setian, Damiani & Dichtchekenian, 1995).
The absence of glycemic control has an important impact on the brain development; the glucose dysregulation can generate several psychological complications not just neuropathies but also risk of cognitive deficits such as of psychological dysfunction. The early childhood is an important time of neuroanatomic progress resulting the fast myelination and maturation into the brain, like so dysglycemia on early childhood has an important impact. The chronic exposure to this clinical condition can affect de development, however there is no concern about that this may occur because the difficult to do a longtime study that can prove the effect during the life. And for this the effects of diabetes type 1 are not fully understood, the neuronal damage can be consequence of the hyper or hypoglycemia, since both can result in consequences to the brain's cells, to comprehend more about that is important to know the type of treatment e al the clinical condition (Duarte, Carvalho, Freire, Hazin, & Arrais, 2012).
It is worth pointing out that the appearance of DM1 during childhood affects the nervous system, which is still developing. This raises the hypothesis that a neuronal nutrition deficit during a sensitive period may cause effects on the neuropsychological development of the child (Kolb & Whishaw, 2002). Some studies report that diabetic children with poorly controlled disease may present great physical and mental fatigue, low motivation, depression, constant inattention and drowsiness (Marcelino & Carvalho, 2005), behaviors that can directly influence the learning processes of these children.
In this sense, studies discuss the modality (which function is more affected) and extent of possible neuropsychological compromises arising from the presence of glucose changes during neurodevelopmental stages. Most of these studies indicate that DM1 can promote significant impact on neuropsychological functioning, especially when we consider three specific variables, which may be combined with each other, namely: a) the young age of the child when diagnosed (Bielssels, Deary & Ryan, 2008; Ohmann et al., 2010); b) exposure to hypoglycemia in children with early onset of disease (Hershey et al., 2010; Lin, Northam, Rankins, Wertherd & Cameron, 2010); c) glycemic control and exposure to hyperglycemia (Duarte et al., 2012; Parent, Wodrich & Hasan, 2009).
Given the above, this study aimed to evaluate aspects of neuropsychological functioning, specifically the intellectual capacity of children with DM1 treated in a public health service located in northeastern Brazil, considering the following clinical variables: a) early onset of disease; b) exposure to episodes of severe hypoglycemia.
METHOD
Participants
This study consisted of an intentional convenience sample of twenty children with DM1. 14 were female and six were male, aged between six and nine years old. It is noteworthy that the choice for this age group was determined in order to investigate the influence of the variable “age of diagnosis”. Studies show variation in the demarcation of the criteria of early onset of disease, ranging from five to seven years old. Thus, the age group of six to nine years includes children with diagnoses which are considered early and late. The maximum age - nine years - was established with the objective of minimizing hormonal influences characteristic of the prepubertal period.
The study was approved by the Ethics Committee of the Federal University of Rio Grande do Norte (UFRN) (report n. 097/2012). The sample of children with DM1 was obtained from the totality of children treated by the pediatric unit of the Pediatric Hospital at the same university. The inclusion criteria for participants were: a) age between six and nine years old; b) diagnosis of DM1; c) treatment by the specialized multidisciplinary clinic for patients suffering from diabetes mellitus; d) Term of Informed Consent (IC) signed by parents and/or guardians. It is important to mention that there is only one reference health service in the city for DM 1, and the total of children registered that met inclusion criteria was 45.
To carry out this study we considered the following clinical variables: a) onset of disease: before six years (early onset) / after six years (late onset); b) glycemic control: appropriate or inappropriate (adequate when being 7-8%, according to the criteria of the Brazilian Diabetes Society), characterized by medical records and consulted on the day of assessment; and c) exposure to episodes of severe hypoglycemia: never, one (1) or two (2) episodes, three (3) or more episodes. The following tables describe the characteristics of the sample according to the clinical variables considered in the study:
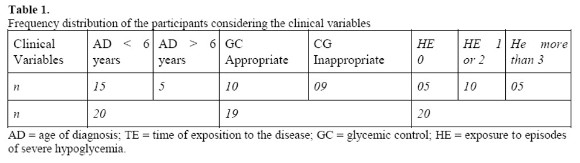
It should be noted that for the variable named glycemic control, only 19 children were considered, since in the medical records of one of the children in the sample had no data that allowed the necessary categorization.
Instrument
The Wechsler Intelligence Scale for Children (WISC-III) was used for evaluating the intellectual abilities of the participants. The test was done in one to two sessions of approximately 50 minutes each. The WISC-III is composed of 13 subtests which, when grouped specifically, provide three IQ scores: Full Scale IQ (FSIQ), Verbal IQ (VIQ) and Performance IQ (PIQ); and four Index Scores: the Verbal Comprehension Index (VCI), the Perceptual Organization Index (POI), the Freedom from Distractibility Index (FDI), and the Processing Speed Index (PSI).
Data Analysis
Initially, we carried out the descriptive analysis of the data obtained with the WISC-III from the total group of children with DM1. The target measurements obtained by the study subjects were contrasted with the test reference measures, respecting the specified criteria (age). Later, inferential analyses were done according to clinical variables (cited above) for testing the significance of the contrasts between the subgroups performance. The effects of the significance of the differences of the performance scores of the groups according to the variables were verified using the non-parametric Mann-Whitney U test for two instances of variation, and the non-parametric Kruskal-Wallis test for more than two instances of variation. It is worth notingto mention that asymmetric distribution of the data was identified.
RESULTS
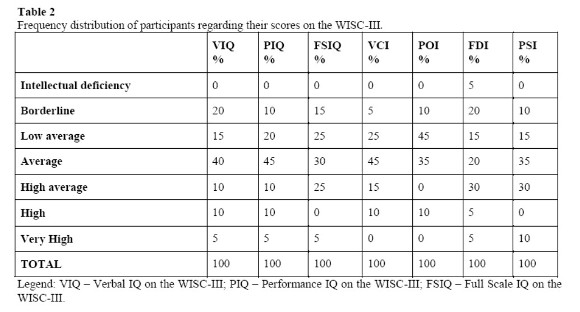
Initially, descriptive statistical analyses were performed based on the performance of the participants on the domains Full Scale IQ, Verbal IQ and Performance IQ, as well as on the Index Scores from the WISC-III subtests. After that, this analysis was extended to each one of the scale's subtests, since the isolated analysis of the IQs is usually not enough for identifying the more subtle and specific deficits. The following table shows the performance of the participants on the WISC-III.
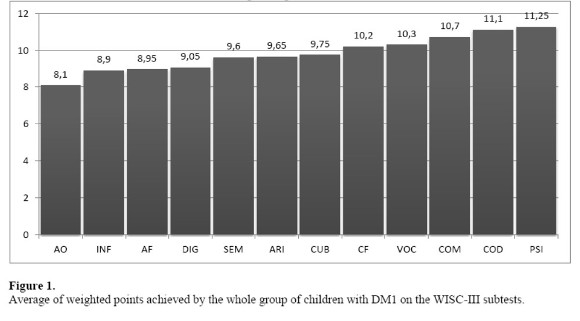
As to the average of weighted points achieved by the whole group of children with DM1 on the WISC-III subtests, we observed the following configuration:
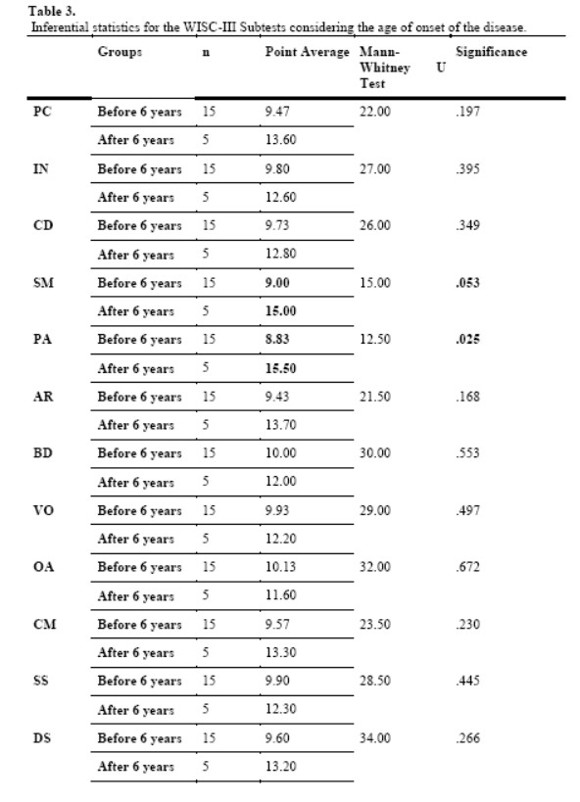
Analyzing the averages obtained on the subtests, we were able to notice that in none of them the global performance of the participants was out of the expected intelligence variation. In a later stage of the analysis, we investigated the interference of the clinical variables which were being assessed (onset of disease, glycemic control, exposure to severe episodes of hypoglycemia) on the performance of the participants. No significant differences were found between the performances of the children when considering the clinical variables “exposure to severe episodes of hypoglycemia”.
The analysis of the variable “age of diagnosis” considered two groups: one of children with early onset of the disease, that is, children diagnosed before they were six years old; and another of children with late onset of the disease, that is, diagnosed after the age of six. According with the global results, a statistically significant difference (p ≤.05) was found in the performance of the groups regarding the subtests Picture Concepts (U = 12.50, p = .02) and Similarities (U = 15.00, p = .05 ). However, when observing the point average obtained with the Mann-Whitney U test, we verified that even though statistical significance was not found, children who were diagnosed after the age of six presented higher average of points on all the other subtests when compared with children with early onset of the disease, as shown on the Table below:
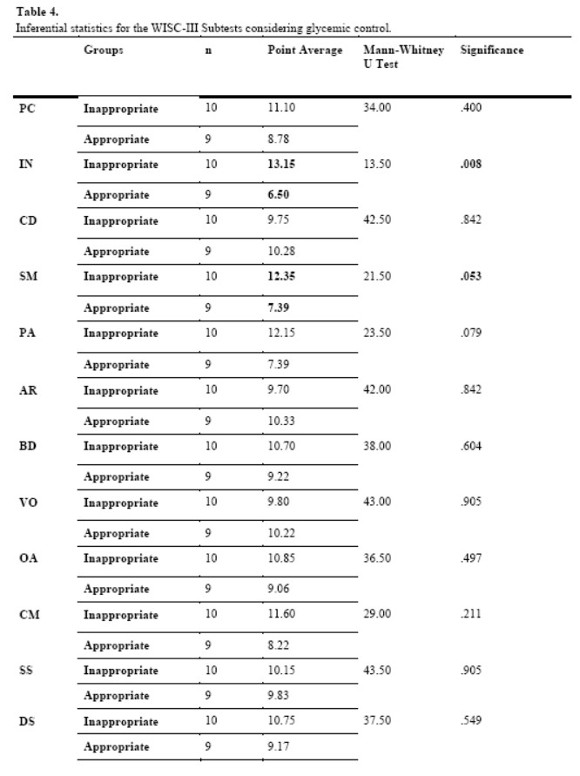
The second clinical variable investigated was “glycemic control”. According to the medical criteria found on the children's records, two instances of variation were established for analyzing glycemic control: appropriate or inappropriate.
When considering the significant interference of the variable in question on the performances of the two groups, we identified statistical significance for the results obtained from the subtests Information (U = 13.50, p = .008) and Similarities (U = 21.53, p = .053).
DISCUSSION
Analyzing the results obtained from the WISC-III by the group of children diagnosed with DM1, we observed that 80 percent of them had a global performance (Full Scale IQ) classified within the normal intelligence range (low average, average, high average). Similar results were found for the performance on the domains Performance IQ (PIQ) and Verbal IQ (VIQ), although the percentage of children who scored within the normal range is 20 percent lower on the latter than on the first one. The same was observed regarding the performance on the Index Scores (Verbal Comprehension Index (VCI), Perceptual Organization Index (POI), Freedom from Distractibility Index (FDI), and Processing Speed Index (PSI)).
This results seems to contradict, in part, the findings by Northam et al. (2001), who, when comparing the performance of children with DM1 to the performance of children with typical development without the disease, found lower scores in children with DM1 for their global performance (FSIQ) on the WISC-III, especially in children who were diagnosed before the age of four and had severe episodes of hypoglycemia. Brands, Biessels, Haan, Kappelle e Kessels (2005), when comparing diabetic and non-diabetic children, found that the former presented lower scores regarding intellectual abilities. Fergunson et al. (2005) found that deficits in non-verbal intelligence skills are common in adults who developed DM1 before the age of seven.
It is important to emphasize that although apparently the results of this study seem to go on opposite directions from those shown in the literature, a more careful analysis shows that from the 20 children studies here, a significant percentage obtained IQ scores placed in the “Borderline” range. This indicates the presence of difficulties on all the intellectual domains which were investigated.
Analyzing the scores obtained by the group of children with DM1 on the WISC-III more closely, that is, focusing on the results from the four Index Scores, we point out that on the Freedom from Distractibility Index (FDI) 5 percent of children had a performance compatible with Intellectual Deficiency, and 20 percent were placed in the Borderline range. Although the average weighted points obtained by the children were situated within the normal range (Arithmetic A = 9.6; Digit Span A= 9.05) – which is due to the presence of 40 percent of children with above the average scores -, it is worth noting that 25 percent of the children in this study performed below the normal range according to the FDI, which suggests the need of investigating the presence of difficulties in the domains of attention and immediate memory in these children more deeply, as well as in the domain of arithmetic, since these domains are required for the execution of the subtests in this score.
It is interesting to note that the literature review did not show data correlating low scores on the FDI with the diagnosis of DM1. The data presented here suggest a fluctuation as to the freedom from distractibility index, probably because of the complex interference of the many clinical variables which permeate the diagnosis of DM1 and seem to be factors which influence the presence of cognitive alterations more than the diagnosis of DM1 alone.
On the other hand, the performance of the children on the Processing Speed Index (PSI) suggests that this is one of the strengths in the cognitive functioning of these children. However, different results have been found in other studies. According to Brands et al. (2005), the presence of DM causes processing speed to slow down. But, for the children in this study, the highest weighted average scores were found on the two subtests of the PSI (Coding A = 11.1 and Symbol Search A =11.25).
Similarly to what was found in the performance analysis of the DM1 group in the tests that make up the PSI, in the Verbal Comprehension Index (VCI) we identified a high percentage of children classified in the middle, with a lower incidence of children in the borderline range (5 percent) and a higher incidence of children categorized as average (45 percent) when compared to other the indices of the WISC-III. Observing the weighted averages obtained in the tests that make up the VCI, it appears that the results obtained in Similarities (A = 9.6), Vocabulary (10.3), Comprehension (10.7) and Information (8.9) are in accordance with the expected average.
The above data suggest that the ability of verbal comprehension is a strength of the cognitive functioning of children in this study. Again, these results are not in accordance with those obtained in other studies. However, we emphasize the difficulty of delimitating the construct verbal intelligence, which obliterates the strict and direct association of this intelligence with the verbal comprehension ability testes by the WISC-III.
Regarding the performance of the group of children with DM1 in the Perceptual Organization Index (POI), we emphasize high frequency with which children were categorized as Lower Average (45 percent), which, added to the Borderline category (10 percent), brings the total of children categorized as below Average to 55 percent. This distribution becomes more relevant when contrasted with the distribution of these categories among the other Index Scores. The sum of the frequency of categories below the Average for the Index Scores was 30 percent for the VCI, 40 percent for the FDI, and 25 percent for the PSI. Thus, it appears that the POI had a higher incidence of performances below Average when compared to the others, and had more than half of the sample situated in this range.
Considering the subtests which compose the POI, we found the following average of weighted points: Object Assembly A = 8.1; Picture Arrangement A = 8.9; Block Design A = 9.75; and Picture Completion A = 10.2. In accordance with these findings, the study by Brands et al. (2005), which is a meta-analysis, found a correlation between children with DM1 and low visual perception performance. This result is similar to that obtained by Fergunson et al. (2005), which found association between DM1 and lower results in non-verbal intelligence tests. Naguib, Kulinskaya, Lomax e Garralda (2009), also in a meta-analysis study, found results in accordance to those mentioned above, since their findings comprehended, among other things, that there was statistical association between lower performances in visuospatial abilities (including the subdomains visual perception and visual motor integration) and a diagnosis of DM1.
Thus, considering the four Index Scores which compose the WISC-III, we observed that the children with DM1 who were evaluated in this research obtained their lowest scores on the Perceptual Organization Index (POI), with 55 percent of the children placed below Average, which indicates that this might be one of the domains which is most affected in this group.
As to the analyses considering the variables delimitated in this research (a) onset of disease; b) glycemic control; and c) exposure to severe episodes of hypoglycemia), we observed statistical significant differences for two of this variables: onset of disease and glycemic control. As mentioned previously, the variable early onset of DM1 is one of the most studied variables because of its correlation with possible neuropsychological effects in these patients.
Regarding the possible influence of this variable, this study found significant statistical differences between children with early onset of DM1 (before the age of six) and late onset (after the age of six) in two subtests of the WISC-III: Picture Concepts and Similarities. However, on all other subtests, even though significant statistical differences were not found, results indicate that children with early onset of the disease had lower average points than children with late onset.
Reinforcing these findings, Biessels et al. (2008) point out that several studies have shown that early onset of DM1 is a strong predictor of damaging impacts on the cognitive functioning of these children. However, these experts point out that the effect of early onset may be mediated by the occurrence of Severe episodes of hypoglycemia. However, this hypothesis was not supported by this study, since the variable exposure to severe episodes of hypoglycemia was not statistically significant in relation to its impact on group performance.
With regard to the worse performance of children with Early Onset on the Similarities subtest of the WISC-III, we bring back the findings by Perantie et al. (2008), which are not in agreement with the results found in this study. In that study, there were no statistical differences with respect to variable Early Onset and the performance of children with DM1 in verbal intelligence tests.
About the results regarding the impact of the clinical variable Glycemic Control on the performance of children with DM1, they should be noted because of the relationship observed between the best performance of children with Inappropriate Glycemic Control. Some studies in the area do not consider this variable in their analyses, stressing that it has no significant impact on cognitive development. This position is supported by Bielssels et al, 2008) and Ohmann et al. (2010), who believe that such impacts would primarily relate to the variable Early Onset.
However, the literature also indicates results in favor of the relationship between the variable Glycemic Control and presence of cognitive impacts, noting that better performances are shown in specific cognitive domains by subjects with Appropriate Glycemic Control (Gonder-Frederick et al., 2009; Knight et al., 2009; Parent, Wodrich & Hasan, 2009; Patiño-Fernández et al., 2010). It is noteworthy that in the studies reviewed there are no results in favor of the group with Inappropriate Glycemic Control, exactly as was observed in this study.
Finally, we go back to the results obtained in the analyses which considered the clinical variable Exposure to Severe Episodes of Hypoglycemia. It is possible to find in the literature that this has been described as an important risk factor when it comes to impact on cognitive performance (Gaudierei et al., 2008). However, we found that there is no consensus as to such an association. Other authors argue that this variable would not bring significant implications for cognitive functioning (Brands et al., 2005; Musen et al., 2008; Patiño-Fernández et al., 2010). There are also a number of researches that emphasize the importance of considering the interaction between this variable and early onset of the disease (Bielssels et al., 2008).
This interaction, in turn, was not observed in this study, since the result of the variable Exposure toSevere Episodes of Hypoglycemia was not statistically significant in relation to its impact on the performance of children, unlike the variable Onset of the Disease.
Thus, in general, this study suggests that the variable Presence of DM1 causes impacts on the cognitive functioning of children with this diagnosis, especially when considering the clinical variable Early Onset of DM1, which is also observed in other studies conducted in the area. In turn, for the results obtained from the clinical variable Glycemic Control by children with DM1, we found that these did not find support in the literature. This finding supports the hypothesis that not only the fluctuation of blood glucose would impact cognition, but the moment of neurodevelopment when the child was exposed to changes in glycemic standards could be more decisive.
This study aimed to investigate the intellectual abilities of children with DM1, considering the interference of clinical variables consecrated in the literature on the performance of these children in order to contribute to the establishment of a neuropsychological profile of children with DM1 treated at a referral center in Rio Grande do Norte, Brazil. The research also contributed to develop the national literature regarding this theme, considering that very few studies were found in Brazil with similar discussions to the ones found internationally.
This study confirmed the results of other studies on the same topic, noting the existence of dissenting conclusions about the neuropsychological findings regarding children with DM1. There is no unanimity in terms of characterization and extent of neuropsychological changes associated with the variables in question. This finding reinforces the need for more studies in this area, especially at a national level, also considering the existing social and economic differences between the various regions of the country that can compromise the functioning of the health services offered, as well as influence access to the information and care needed for monitoring individuals who have the disease.
We also suggest that future research with the same character contemplate neuroimaging, in order to make associations between neuropsychological functioning, neurodevelopmental data and brain structure. It would also be relevant to include the observation of children with DM1 in their school environment and their academic performance.
The knowledge of how cognitive functioning operates on the interface with disease, considering the different clinical and socio-demographic related variables, such as time of exposure of the child to the disease, are indispensable to the creation and implementation of early interventions strategies that minimize possible impacts of the disease and even overcome cognitive difficulties there are specific to this clinical group.
We hope that future studies overcome these limitations and address the advances provided here. We also expect that this study will contribute to further reflections on the neuropsychological functioning of children with DM1, especially drawing attention to neurodevelopmental aspects that are inherent to this discussion and serve as aids to the development of interventions that address the necessary care for the full development of these children.
REFERENCES
Alves, C., Veiga, T., Toralles, M., & Ribeiro, F. (2008). Outpatient follow-up for children and adolescents with diabetes mellitus type 1 in the city of Salvador. Revista Baiana Saúde Pública, 31, 52-67. Retirado de http://inseer.ibict.br/rbsp/index.php/rbsp/article/viewFile/1390/1026 [ Links ]
Bielssels, G. J., Deary, I. J., & Ryan, C. M. (2008). Cognition and Diabetes: a lifespan perspective. Lancelet Neurology, 7, 184-190. doi: 10.1016/S1474-4422(08)70021-8 [ Links ]
Brands, A. M. A., Biessels, G. J., Haan, E. H. F., Kappelle L. J., & Kessels, R. P. C. (2005). The Effects of Type 1 Diabetes on Cognitive Performance. Diabetes
Care, 28, 726 – 735. doi: 10.2337/diacare.28.3.726
Duarte, N. S., Carvalho, M. F. O., Freire, R. C. L., Hazin, I., & Arrais, R. F. (2012). Type 1 diabetes mellitus and neuropsychology abilities: literature review. Journal of Nursing UFPE on line, 63032-3040. doi: 10.5205/reuol.2255-18586-1-LE.0607201221 [ Links ]
Ferguson, S. C., Blane, A., Wardlaw, J., Frier, B. M., Perros, P., McCrimmon, R. J., et al. (2005). Influence of an Early-Onset Age of Type 1 Diabetes on Cerebral Structure and Cognitive Function. Diabetes Care, 28, 1431 – 1437 [ Links ]
Gan, M. J., Albanese-O'Nell, A., & Haller, M.J. (2012). Type 1 diabetes: current concepts in epidemiology, pathophysiology, clinical care and research. Current Problems Pediatric Adolescent Health Care,42 , 269-91. doi: 10.1016/j.cppeds.2012.07.002 [ Links ]
Gaudierei, P. A., Chen, R., Greer, T. F., & Holmes, C. S. (2008). Cognitive Function in Children With Type 1 Diabetes. Diabetes Care, 31, 1892–1897. (Duarte, Carvalho, Freire, Hazin & Arrais, 2012). doi: 10.2337/dc07-2132 [ Links ]
Gonder-Frederick, L. A., Zrebiec, J. R., Bauchowitz A.U., Ritterband, L. M., Magee, J. C., & Cox, D. J. (2009). Cognitive function is disrupted by both hypo- and hyperglycemia in school-aged children with type 1 diabetes: a field study. Diabetes Care, 32, 1001-1006. doi: 10.2337/dc08-1722 [ Links ]
Hershey, T., Perantie, D. C., Wu, J., Weaver, P. M., Black, K. J., & White, N. H. (2010). Hippocampal Volumes in Youth With Type 1 Diabetes. Diabetes, 59, 236 – 241. doi: 10.2337/db09-1117 [ Links ]
Klafke, A., Duncan, B. Bartholow, R., Moura, R.S., Malta, L., Carvalho, D., & Schmidt, M.I. (2014). Mortalidade por complicações agudas do diabetes melito no Brasil, 2006-2010. Epidemiologia e Serviços de Saúde, 23, 455-462. doi: 10.5123/S1679-49742014000300008 [ Links ]
Knight, S., Northam, E., Donath, S. Gardner, A., Harkin, N., & Taplin, C., (2009). Improvements in cognition, mood and behavior following commencement of continuous subcutaneous insulin infusion therapy in children with type 1 diabetes mellitus: a pilot study. Diabetologia, 52, 193–198. doi: 10.1007/s00125-008-1197-3 [ Links ]
Kolb, B., & Whishaw I. Q. (2002). Neurociência do comportamento. São Paulo: Manole. [ Links ]
Lin, A., Northam, E. A., Rankins, D., Wertherd, G. A., & Cameron, F. J. (2010). Neuropsychological profiles of young people with type 1 diabetes 12 year after disease onset. Pediatric Diabetes, 11, 235– 243. doi:10.2337/diacare.24.9.1541 [ Links ]
Marcelino, D. B., & Carvalho, M. D. B. (2005). Reflexões da Diabetes tipo I e sua relação com o Emocional. Psicologia Reflexão e Crítica, 18, 72-77. doi: 10.1590/S0102-79722005000100010 [ Links ]
Musen, G., Jacobson, A.M., Ryan, C. M., Cleary, P. A., Waberski, B. H., & Weinger, K., (2008). Impact of Diabetes and Its Treatment on Cognitive Function Among Adolescents who Participated in the Diabetes Control and Complications Trial. Diabetes Care, 31, 1933 – 1938. doi:10.2337/dc08-0607 [ Links ]
Naguib, J. M., Kulinskaya, E., Lomax, C. L., & Garralda, M. E. (2009). Neuro-cognitive Performance in Children with Type 1 Diabetes—A Meta-analysis. Journal of Pediatric Psychology, 34, 271-282. doi: 10.1093/jpepsy/jsn074 [ Links ]
Northam, E. A., Anderson, P. J., Jacobs, R., Hughes, M., Lwarne, G., & Werther, G. A. (2001). Neuropsychological Profiles of Children With Type 1 Diabetes 6 Years After Disease Onset. Diabetes Care, 24, 1541-1546. [ Links ]
Ohmann, S., Popow, C., Rami, B., Konig, M., Blass, S., Fliri, C., & Shober, E. (2010). Cognitive function and glycemic control in childrens and adolescents with type 1 diabetes. Psychological Medicine, 40, 95-103. doi: 10.1017/S0033291709005777 [ Links ]
Patiño-Fernández, A.M., Delamater, A. M., Applegate, E. B., Brady, E., Eidson, M., & Nemery, R. (2010). Neurocognitive functioning in preschool-age children with type 1 diabetes mellitus. Pediatric Diabetes, 11, 424–430. doi:10.1111/j.1399-5448.2009.00618.x [ Links ]
Parent, K. B., Wodrich, D.L., & Hasan, K. S. (2009). Type 1 diabetes mellitus and school: a comparison of patients and healthy siblings. Pediatric Diabetes, 10, 554–562. doi: 10.1111/j.1399-5448.2009.00532.x [ Links ]
Patterson, C.,Guarigata, L., Dahlquist, G., Soltész, G., & Silink, M. (2014) Diabetes in the young – a global view and worldwide estimates of numbers of chlidren with type
1 diabetes. Diabetes Research and Clinical Practice,.103,161-75. doi: 10.1016/j.diabres.2013.11.005
Perantie, D. C., Lima, A., Wua, J., Weaver, P., Warrenb, S. L., & Sadler, M. (2008). Effects ofprior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatric Diabetes, 9, 87– 95. doi: 10.1111/j.1399-5448.2007.00274 [ Links ]
Setian, N., Damiani, D., & Dichtchekenian, V. (1995). Diabetes mellitus na Criança e no Adolescente: encarando o desafio. São Paulo: Sarvier. [ Links ]
Endereço para Correspondência
Campus Universitário Lagoa Nova, Av. sem. Salgado Filho, 3000, Candelária, Natal/RN – Brasi/CEP 59066-800. Telf.: . E-mail: izabel.hazin@gmail.com
Recebido em 27 de Outubro de 2015
Aceite em 16 de Março de 2017














