INTRODUCTION
Physical exercise is a known factor that can promote oxidative stress and may result in cellular damage if not neutralized by antioxidant mechanisms (Ramos et al., 2013). There has been increasing interest in the investigation of high-intensity interval training (HIIT), as indicated not only by the number of scientific publications (Evangelista et al., 2017). There is an accumulation of evidence (Bogdanis et al., 2013; Burgomaster et al., 2008; Gibala et al., 2012; Gillen et al., 2016) that HIIT training protocols promote physiological stimuli comparable to continuous endurance training, despite a substantially shorter time commitment and reduction of total exercise volume. However, there is still no consensus regarding HIIT's impact on oxidative stress (Halliwel, 1996).
Oxidative stress is the imbalance between the oxidizing process and intracellular antioxidants (Halliwel, 1996; Powers & Nelson, 2011; Rosa-Lima et al., 2015). The main causative agents are free radicals (FR), which are unstable molecules generated from reactive oxygen species (ROS) (Halliwel, 1996; Powers & Nelson, 2011; Rosa-Lima et al., 2015). The ROS can alter redox homeostasis, causing oxidative stress and damaging macromolecules, proteins, and DNA (Pisoschi & Pop, 2015; Sies & Jones, 2007; Wadley et al., 2016). However, physical exercise appears to be the primary regulator of the redox state (Power & Jackson, 2008).
The various oxidative stress and cellular damage indicators are measured mainly in plasma samples (Ramos et al., 2013; Wadley et al., 2016; Power & Jackson, 2008 Fisher-Wellman et al., 2009; Valko et al., 2007). ROS's excessive production is reduced or controlled by several enzymatic and non-enzymatic antioxidants that prevent the oxidative damage of biological membranes (Powers & Nelson, 2011). However, there is still no consensus on which tissues undergo significant oxidative damage in response to high-intensity exercise.
Surprisingly, there is little information available about oxidative damage in hepatic tissue since most studies are conducted on muscle tissue (Ramos et al., 2013). Furthermore, the effects of consecutive and non-consecutive sessions of HIIT on the markers of hepatic oxidative damage are not yet fully elucidated. Thus, the objective of this study was to evaluate the effects of 12 consecutive and non-consecutive sessions of HIIT on markers of hepatic oxidative stress in rats.
METHOD
Animals
Thirty-two rats (Rattus Norvegicus, Wistar lineage) weighing 250 to 300g and 7 to 8 weeks of age were used. The animals were randomly and equally divided into four groups with eight animals each as follows: sedentary control group (CS1); group trained with HIIT for 12 continuous sessions totaling 12 days (CT1); group trained with HIIT for 12 sessions, three times a week, totaling 4 weeks (CT2); and a second sedentary control (CS2). The animals were kept in collective cages in groups of four rodents under ambient temperature conditions of 21 °C to 24 °C, and 42%-50% humidity, monitored by a Hikari HTH-240 Digital Thermo-Hygrometer (Hikari, China), and a 12-hour light-dark cycle with free access to filtered water and rodent-specific feed (Purina®). The procedures that were used in this study were previously approved by the Animal Research Ethics Committee of the Federal University of Sergipe (CEPA/UFS) under the protocol (15/2017) and were in accordance with the Guidelines of the Brazilian College of Animal Experiments (COBEA).população.
Procedures
Training protocol
The rats were submitted to swimming training, which consisted of 14 swimming intervals with a duration of 20 seconds each and a pause of 10 seconds between each interval. A load of 14% of body weight was used with a water level of 60 cm. The CT1 group performed the HIIT for 12 consecutive days, and the CT2 group performed the HIIT intermittently (three times a week) for 4 weeks. After each swimming training session, all the animals were dried to avoid cold and moisture physiological complications. Before the training session, adaptation to the water was performed for 3 weeks, and CS1 and CS2 groups were submitted only in the first week of adaptation to the liquid medium, following the protocol adapted from the study by Contarteze et al. (2007), in a cylindrical tank 80 cm deep x 80 cm in diameter and water temperature of 25 °C ± 1 °C with lead overload (small cotton fabric bags and Velcro®) tied to the back. All animals also underwent an adaptation protocol in the tanks (80 cm depth x 80 cm in diameter) with different levels of water, load, exercise intensity, and water volume (Table 1).
Euthanasia
The animals were anesthetized with ketamine/xylazine (75mg/kg + 10mg/kg i.p.), and the blood (approximate amount of 5mL) was collected through a cardiac puncture 24 hours after the last session of the experimental period, which was 12 days for CS1 and CT1, and 4 weeks for CS2 and CT2. Immediately after collection, the blood was centrifuged at 800 g for 15 minutes at 4 °C. The supernatant was then stored at −80 °C. The liver was removed and then washed three times with KCl 1.15% solution (Vetec, LTDA, Rio de Janeiro, Brazil). Immediately after the liver was homogenized, each gram of the tissue was mixed with 5 mL of KCl, 10μL of phenylmethylsulfonyl fluoride (PMSF, 100mmol, Sigma-Aldrich, Steinheim, Germany), and 15μL of 10% Triton. The homogenate was then centrifuged at 3000 g for 10 minutes at 4 °C. The supernatant was stored at −80 °C until further analyzed for oxidative stress and tissue damage markers.
This procedure was done to measure the lipid peroxidation products through thiobarbituric acid reactive substances (TBARS). The TBARS measurement was performed according to the method described by Ohkawa et al. (1979). One-hundred microliters of heparin homogenate were incubated in Eppendorf tubes with 350 μL of 20% acetic acid (pH 3.5) and 600 μL of thiobarbituric acid (TBA, 0.36%) for 1 hour at 85 °C to quantify the TBARS. The Eppendorfs were then cooled on ice and centrifuged at 1500 g for 5 minutes.
The absorbance reading was performed at 532 nm. Tetramethoxypropane (TMP) was used as an external standard, and the level of lipid peroxides was expressed in μmol TARS/mg protein, where TBARS stands for thiobarbituric acid reactive substances.
Determination of antioxidant capacity
Using the Ferric Reducing Ability of Plasma (FRAP) technique, a 9 μL aliquot of liver plasma was pipetted onto a microplate where 27 μL of distilled water and 270 μL of the FRAP reagent were added. The plate was incubated at 37 °C for 30 minutes and the reading performed at 595nm. Ferrous sulfate (FeSO4) was used as the standard, and the results are expressed in μM ferrous sulfate equivalents produced (Singhal, Paul & Singh, 2014).
Determination of total sulfhydryl (thiols)
Total sulfhydryl was determined according to the methodology described by Faure and Lafond (1995). Aliquots of 50 μL of the sample (liver) were mixed in 1.0 mL of Tris-EDTA buffer, pH 8.2. Next, the first reading (A) was obtained using a spectrophotometer at 412 nm. After reading, the samples were transferred to assay tubes and blended in 20 μL of 10 mM DTNB (Ellman’s Reagent) diluted in methanol (4 mg/mL), under dark conditions. At the end of 15 minutes, the second absorbance reading (A2) was obtained. The sulfhydryl (SH) concentration was calculated according to the equation
Plasma analyses
For the measurement of uric acid (enzymatic UV uricase-peroxidase), a commercial kit (Labtest®, Minas Gerais, Brazil) was used. Plasma (20 μL) of each animal was homogenized in kit-specific reagents at 37 °C ± 0.2 °C, and the readings were obtained using a spectrophotometer (BioSpectro Model SP-22 UV/Visible, Minas Gerais, Brazil) at a wavelength of 540 nm.
The quantification of the liver-tissue specific damage caused by HIIT was assessed by measuring the tissue enzymatic markers such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST). For quantification, a commercial kit (Labtest®, Minas Gerais, Brazil) was used. Plasma (20 μL) of each animal was homogenized in kit-specific reagents at 37 °C ± 0.2 °C, and the readings were obtained using a spectrophotometer (BioSpectro Model SP-22 UV/Visible, Minas Gerais, Brazil) at a wavelength of 340 nm.
Statistical analysis
The central tendency measures, mean ± standard deviation (X ± SD), were used. The Shapiro Wilk test was used to test variables' normality considering the sample size. To verify the possible differences between the groups, the two-way ANOVA test with Bonferroni post hoc test was used. In order to verify the size of the effect, the Cohen f2 test was used, in addition to the cut-off points 0.02 to 0.15 for a small effect, 0.15 to 0.35 for medium effect, and greater than 0.35 for large effect, according to Grissom and Kim (2005). The statistical analysis was performed using the Statistical Package for Social Science (SPSS), version 22.0. Alpha value was set at .05.
RESULTS
The parameters analyzed are shown in Figures 1, 2, 3, 4, 5, and 6.
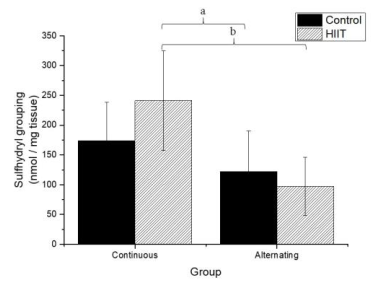
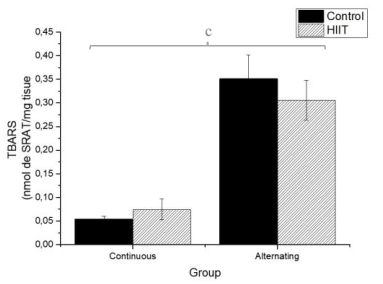
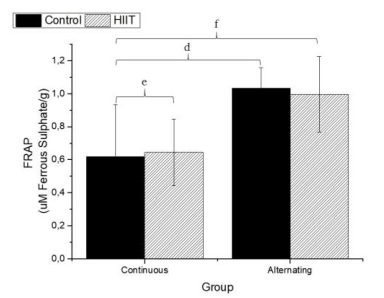
There were significant differences regarding the sulfhydryl groups (Figure 1) between the alternate control group (CS2) and continuous HIIT group (CT1) (a: p = 0.009), and there were differences between continuous training HIIT (CT1) and alternate training HIIT (CT2) (b: p = 0.001). Regarding sulfhydryl, there was a large effect (Cohen's f2 = 0.434). Regarding TBARS (Figure 2), there were significant differences between the continuous control group (CS1) and alternate control group CS2, CS1 and the alternate HIIT group CT2, and CT1 and CT2 (c: p < 0.001), and the effect was large (f2 of Cohen = 0.943). Regarding FRAP (Figure 3), there were significant differences between CS1 and CS2 (d: p = 0.006), CS1 and CT2 (e: p = 0.015), and continuous HIIT CT1 and CS2 as well as CT2 (f: p = 0.017), with a large effect (Cohen's f2 = 0.450).
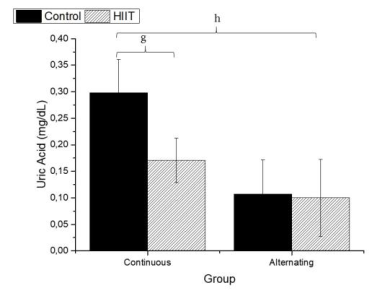
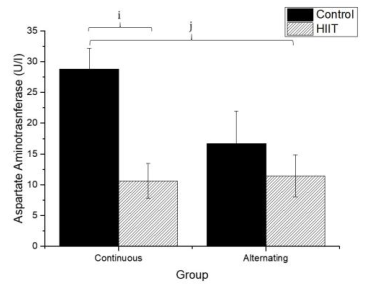
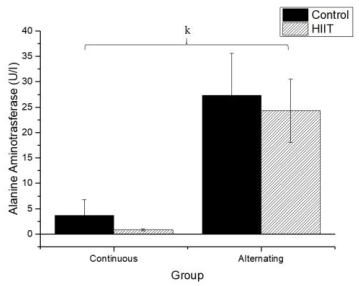
Regarding uric acid (Figure 4), there were significant differences between CS1 and CT1 (g: p = 0.002) and between CS2 and CT2 (h; p < 0.001), with a large effect (Cohen's f2 = 0.655). Regarding AST (Firugre 5), there was a significant difference between CS1 and CT1 (i: p = 0.038) and between CS2 and CT2 (j: p = 0.001), with a large effect (Cohen's f2 = 0.472). Regarding ALT (Figure 6), there were significant differences between all groups (k; p < 0.001), with a large effect (Cohen's f2 = 0.745).
DISCUSSION
The main finding from this study was that HIIT for 12 consecutive or 12 non-consecutive days over 4 weeks did not cause liver damage as indicated by measures evaluated. From the TBARS method, it was possible to detect oxidative damage in biomolecules based on the degree of lipid oxidation (Ayala, Muñoz & Arguelles, 2014). Corroborating the findings of the present study, Songstad et al. (2015) did not detect a significant difference in lipid peroxidation, but the model was running on the treadmill for 15 non-consecutive sessions. Casimiro-Lopes et al. (2012), using the same protocol as Terada et al. (2001) but for 24 sessions, also did not find significant alterations in lipid peroxidation. However, previous studies have shown that high-intensity exercises cause more lipid peroxidation compared with moderate or low-intensity exercises (Balci & Pepe, 2012).
According to Azizbebeigi et al. (2014), high-intensity exercise for preventing free radical damage acts by strengthening the antioxidant defense system. However, this does not seem to be the case in the present study or in a study by De Araújo et al. (2016) who submitted rats to jumps in the water for 36 and 72 sessions and did not find significant differences in the production of reactive species and oxidative stress.
Damage to cell membranes can release cell plasma contents after strenuous exercise (Kalafati et al., 2010). This condition is identified in the liver's case by the release of intracellular enzymes such as ALT and AST (Ramos et al., 2013), which are considered the most important hepatic enzymes (Altinoz et al., 2016). However, these enzymes have no known physiological function in plasma, and their quantifications may provide information on the location of the damage (liver tissue) (Chen & Hsieh, 2001; Skenderi et al., 2006; Skenderi et al., 2008).
It has been fully established that hepatic lesions are accompanied by changes in the plasma concentration of hepatic enzymes, including AST and ALT (Xie et al.,2013). The higher the concentrations of hepatic enzymes, the greater the risk of metabolic syndromes (Whitfield et al., 2013). In the present study, no significant differences were found in AST and ALT levels. Previous data support our findings, such as those of Dos Santos et al. (2014), who used a high-intensity resistance training model for 12 sessions over 4 weeks. Also, Motta et al. (2016) used a 20-second exercise protocol with 10 seconds pause in rats and found a significant decrease in AST and ALT levels after 36 swim sessions performed three times a week for 12 weeks, with the addition of weights starting at 10% of body weight and ending at 15% of body weight. Another study by Righ et al. (2016), who used a moderate-intensity exercise, did not show differences in these markers between the control and trained groups, thus providing further evidence that HIIT does not cause hepatic damage.
Depending on the type of exercise, the muscles undergo enzymatic extravasation of uric acid and consequent accumulation in plasma. FRAP is a parameter of antioxidant activity in plasma and is determined by the blood's uric acid content (Balogh et al., 2001). The increase in plasma uric acid after intense exercise may indicate an increase in the antioxidant defense capacity (Baker et al., 2004; Chatzinikolaou et al., 2010). As we can see from the data obtained, there were no significant differences in uric acid level or FRAP in the test groups compared with the control groups. Although we did not observe significant differences in the FRAP antioxidant system, Freitas et al. (2018) evaluated HIIT's effects on treadmill running rats during 36 non-consecutive sessions. They observed a significant increase in the HIIT group compared to the control. The muscle damage from running is likely more extensive than that induced by swim training.
CONCLUSION
HIIT performed on consecutive (12 sessions) or alternating days (12 sessions, three times a week for 4 weeks) did not promote hepatic damage, as indicated by markers of oxidative stress and plasma levels of liver enzymes, in rats. It is worth noting that these parameters should be evaluated in other tissues to guarantee greater safety in maintaining health.