INTRODUCTION
Flexibility is an important component of the physical fitness of athletes, non-athletes, and those from different populations engaged in physical activity, such as children, youth, adults, and the elderly (Dantas & Conceição, 2017). Despite the global use of stretching exercises, studies on the forces applied to joints during these training routines remain scarce (Jacobs & Sciascia, 2011; Apostolopoulos, Metsios, Nevill, Koutedakis, & Wyon, 2015a; Gerdijan, Perić, Ljubojević, & Vukić, 2021).
The frequency, intensity, and duration of stretching exercises are considered flexibility training parameters (Apostolopoulos, 2018). However, in contrast to duration and frequency, which are quantifiable, intensity is a qualitative variable that has received little scientific interest (Apostolopoulos et al., 2015a). Intensity is the magnitude of force or torque applied to the joint during stretching exercises. Low force may result in an elastic response with little or no gain in the range of motion. By contrast, applying significant force may injure tissue, causing an inflammatory response (Jacobs & Sciascia, 2011). This inflammatory response to exercise-induced tissue damage is characterised by leukocyte infiltration and proinflammatory cytokine production in the injured muscle tissue (Deyhle et al., 2016).
Previous studies suggest that the intensity and duration of exercise (Silva & Macedo, 2011; Pedersen, 2019), type of exercise and type of contraction (for example, eccentric or concentric), tissue damage and the muscle mass involved (Pedersen, 2019) influence the interleukin 6 (IL-6) response to acute exercise. The release of IL-6 initiates a systemic response, with changes in the acute phase, which reflect the presence and intensity of inflammation, with an increase in C-reactive protein (CRP) proportional to the inflammatory stimulus (Apostolopoulos, Metsios, Taunton, Koutedakis, & Wyon, 2015b).
The impact of flexibility training on inflammation has been little investigated in the scientific literature, with few studies conducted with animals (Pizza, Koh, McGregor, & Brooks, 2002; Berrueta et al., 2016) and humans (Apostolopoulos et al., 2015a; 2015b; 2018). Experiments with mice reported high levels of neutrophils after an intense passive stretching protocol, representing an acute inflammatory response since activated neutrophils secrete proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 (Pizza et al., 2002). Human studies have shown significant increases in hs-CRP levels after maximal stretching compared to submaximal stretching (Apostolopoulos et al., 2015a) and 24h after maximal stretching vs control (Apostolopoulos et al., 2015b). However, only one of these studies (Apostolopoulos et al., 2015a) analysed the effects of stretching intensity on inflammation, in which the authors evaluated a single inflammatory biomarker.
Studying changes in inflammatory responses following stretching could complement the information provided by traditional markers already evident in the literature such as creatine kinase (CK) or delayed onset muscle soreness (DOMS) (Apostolopoulos et al., 2018). Furthermore, the ability to quantify the physiological impact of a stretching exercise session through the analysis of inflammatory biomarkers is crucial to understanding recovery needs, and allowing adequate rest before a second exercise session (Lee et al., 2017). Therefore, the present study analysed the acute effects of applying flexibility training with different intensities on the inflammatory responses of young military personnel. The experimental hypothesis suggested that applying maximal stretching intensity would promote greater changes in the inflammatory markers CRP, IL-6, and IL-10 when compared to stretching applied at submaximal intensities.
METHODS
Study design
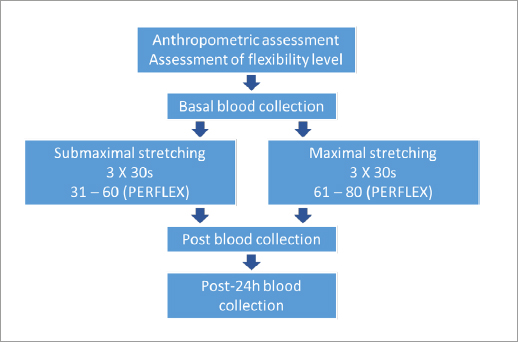
This factorial study (2 groups × 3 moments) investigated the effects of the intensity of flexibility training sessions (submaximal vs. maximal) on the inflammatory response in young military personnel. All the procedures were conducted at the Air Force Cadet Preparatory School (EPCAR) over three consecutive days. On the first visit to the laboratory, an anthropometric assessment was performed to characterise the sample, and the participants’ flexibility level was assessed by the LABIFIE goniometric protocol (Dantas, Carvalho, & Fonseca, 1997) to determine if all the participants exhibited a healthy range of motion (ROM). Participants performed a flexibility exercise routine on the second visit with maximal or submaximal intensity. The visits were standardised between 4 and 6 pm (during the students’ physical education instruction). Blood samples were collected pre (baseline), immediately post, and 24 h post-exercise (third day) to quantify the inflammatory markers CRP, IL-6, and IL-10 (dependent variables). The independent variables were the intensity of flexibility training (submaximal and maximal) and the timepoint of measurements. Figure 1 illustrates the experimental design and timeline.
The study was disseminated at a lecture given to the military personnel one week before the procedure. After instruction, subjects were invited to take part in the study voluntarily. All the individuals had participated in a continuous military physical training program for at least one year and were familiar with flexibility exercises.
Participants
Thirty young trained male military personnel between the ages of 18 and 19 years volunteered for the study. The sample size was calculated using G Power software, Version 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007), which revealed a minimum number of 28 individuals, according to the study design, for a statistical power of 0.80 and estimated effect of 0.2 (Beck, 2013).
Using a randomisation program (https://www.randomizer.org/), participants were equally assigned to one of two groups: a) submaximal and b) maximal stretching. No differences in anthropometric characteristics and flexibility levels were found between groups (Table 1). Inclusion criteria consisted of members of the military physical training team (at least one year) who were experienced in flexibility training and had medical clearance to perform exercises according to the Air Force Health Inspection (ICA 160-6/2016) and answered negatively to all the Physical Activity Readiness Questionnaire (PAR-Q) questions (Shephard, 1988). Individuals with any acute or chronic health conditions, musculoskeletal injuries that compromised their ability to perform physical activities, were on any drugs, medication, or dietary supplements altering their metabolism, as well as anti-inflammatories (i.e., NSAIDs) were excluded from the study. This study was approved by the Local Committee of Ethics in Research (protocol no. 20106719.9.0000.5285) and conducted in accordance with the ethical principles of the Declaration of Helsinki. Participants provided written informed consent to participate in the study.
Table 1 Anthropometric characteristics and average joint range of motion (in degrees) of the individuals.
| Submaximal Stretching (n= 14) | Maximal Stretching (n= 14) | p-value | |
|---|---|---|---|
| Age (years) | 18.29= 0.47 | 18.21= 0.43 | 0.676 |
| Body mass (kg) | 70.65= 7.87 | 67.19= 4.46 | 0.164 |
| Height (cm) | 174.66= 4.93 | 173.18= 4.95 | 0.436 |
| Body fat (%) | 11.36=3.83 | 10.01= 2.41 | 0.272 |
| SHF (º) | 125.72= 4.78 | 128.92= 4.55 | 0.081 |
| SHE (º) | 66.36= 12.23 | 65.62= 14.54 | 0.885 |
| HF (º) | 100.69= 7.94 | 101.29= 8.28 | 0.846 |
| HE (º) | 42.26= 9.62 | 44.63= 10.57 | 0.540 |
*Data presented as mean± SD; SHS: Shoulder horizontal flexion; SHE: Shoulder horizontal extension; HF: Hip flexion; HE: Hip extension.
After the experimental procedure, two participants were excluded from the initial sample (one from each group) since they had an acute infection, which resulted in IL-10’s basal values higher than the reference values (< 9.1 pg/mL). Therefore, the data analysis consisted of 28 military personnel (18.25± 0.44 years, 68.92± 6.52 kg, 173.92± 4.91 cm) equally randomised (14 per group) into two groups.
Anthropometric assessment and flexibility level
The anthropometric assessment was performed, including measurements of body mass (kg) (Filizola, ID-M 150/4, São Paulo, Brazil), height (cm) (Sanny, Standard, São Bernardo do Campo, Brazil), and chest, abdomen, and thigh skinfolds thickness (mm) using a skinfold calliper (Cescorf, Top Tec, Porto Alegre, Brazil). The Jackson and Pollock (1978) equation (Jackson & Pollock, 1978) was used to calculate the body density, and the fat percentage was estimated using the Siri equation (1961) (Siri, 1993). After that, the participants’ flexibility level was assessed by the LABIFIE goniometric protocol (Dantas et al., 1997). Shoulder horizontal flexion and extension (SHF/SHE), as well as hip flexion and extension (HF/HE) movements, were assessed using a Medigauge digital goniometer, USA (www.medigauge.com).
Experimental routine
In the submaximal stretching group (SG) (n= 14), participants performed a passive static stretching routine for the upper and lower limbs, consisting of three 30-second sets of submaximal intensity (level 31-60 on the PERFLEX scale) (Dantas et al., 2008) followed by a 10-second interval between them. Participants of the maximal stretching group (MG) (n= 14) performed a maximal intensity passive static stretching routine, at a discomfort level equivalent to 61-80 on the PERFLEX scale (Dantas et al., 2008), for the upper and lower limbs consisting of three 30-second sets followed by a 10-second interval between them. The interval rest length between sets was defined according to two previous studies (Apostolopoulos et al., 2015a; Apostolopoulos, 2018).
The Perceived Exertion in Flexibility (PERFLEX) Scale was used to assess perceived exertion during the exercises (Dantas et al., 2008). The scale, which consists of five intensity levels varying from 0 to 110, is categorised into five verbal descriptors (Table 2), allowing the participants to rate the sensation in accordance with the ROM performed. In both experimental conditions, the participants’ perceived exertion was monitored throughout the sets to ensure that the appropriate intensity was maintained.
Table 2 Perceived Exertion Scale of Flexibility (PERFLEX).
| Level | Description of the sensation | Effect | Specification |
|---|---|---|---|
| 0-30 | Normality | Mobility | No change whatsoever in mechanical, plastic and inextensible components. |
| 31-60 | Forcing | Stretching | Causes deformation of plastic components and the elastic components are stretched to the submaximal level. |
| 61-80 | Discomfort | Flexibilizing | Causes lasting adaptations in the plastic, elastic and inextensible components. |
| 81-90 | Bearable Pain | Possible injury | The muscle-conjunctive structures involved are subjected to extreme stretching, causing pain. |
| 91-110 | Strong Pain | Injury | Exceeds the extreme stretching of structures involved focusing mainly on the skeletal |
Flexibility exercise protocol
The present study used the static stretching method since it is easy to perform and is commonly used in clinical and sports practise (Nogueira et al., 2020). The same researcher applied the flexibility exercises bilaterally over a single session. The exercises are described below:
Shoulder horizontal flexion (SHF): With participants standing upright, head facing forward, shoulders symmetrical, with arms abducted at a 90º angle, and trunk and elbows extended with the palms of the hands facing downward, the examiner horizontally flexes the shoulder;
Shoulder horizontal extension (SHE): With the participant’s position being similar to the SHF exercise, instead of the examiner horizontally flexion the shoulder, they horizontally extend it;
Hip flexion (HF): With participants in dorsal decubitus with arms extended backwards alongside the head with one of the knees extended, the examiner placed his right hand on the participant’s leg extended on the floor, preventing it and the hip from rising. With his left hand on the knee of the other leg, the examiner moved it towards the trunk;
Hip extension (HE): with participants ion in ventral decubitus, with hands extended forward, the examiner pushed down on the individual’s hip with one hand and placed the other hand under the knee, raising it and extending the hip.
To analyse the isolated effect of stretching exercises on inflammation, we did not conduct warm-up with other exercises before the intervention.
All the volunteers exhibited the same dietary pattern one week before the experimental procedure and on the collection days, controlled by the nutritionist team of the military organisation.
In order to guarantee balanced hydration during exercise, each individual was instructed to drink 5 to 7 millilitres of water per kilogram of bodyweight upon awakening on collection days (Thomas, Erdman, & Burke, 2016). The aim was to reduce the possible harmful effects on physical performance and avoid inconsistent haematological basal parameters caused by a possible state of dehydration. All the study participants abstained from alcohol for 48 hours before the procedures.
Blood collection, movement, storage, and disposal
With the use of a vacutainer, approximately 15 ml of blood was collected from an antecubital vein of each participant at pre (basal), immediately post, and 24 h post-exercise by an experienced nurse. To prevent puncture damage to the participant’s veins, arms were alternated for collection. The blood samples were immediately allocated into two 5 ml vacutainer tubes (Becton Dickinson, Juiz de Fora, Brazil) containing EDTA for plasma separation and into one 5 ml dry vacutainer tube for serum separation. The CRP samples were centrifuged for 10 minutes at 3000 rpm and stored at temperatures between 2 and 8°C (refrigerated). After centrifugation and visual analysis (hemolysis, lipemia, jaundice), the tubes were placed in racks and taken for analysis. The IL-6 and IL-10 samples were centrifuged for 10 minutes at 3,000 rpm and stored at 0°C (frozen). Thereafter, the samples were sent to the laboratory, where they were stored at -80°C for subsequent analyses.
CRP analysis was conducted using the latex-enhanced immunoturbidimetric assay. The IL-6 analysis was carried out using the IL-6 kit (Lote: 410; Supplier: Siemens; Device: Immulite 2000 XPI). Analysis for IL-10 was conducted using the IL-10 kit (Lot: 412 Supplier: Siemens; Device: Immulite 1000. IL-6 and IL-10 analyses were carried out using the chemiluminescence method. For IL-6 and IL-10, analytical sensitivity was < 2 and < 5 pg/mL, respectively. The samples of serum concentrations of IL-6 and IL-10 were analysed in duplicate, with inter/intra-assay coefficients of variation (CV) less than 3%.
Statistical analysis
The values are presented as mean and standard deviation (Mean± SD). The Shapiro-Wilk test was used to analyse the normality of the variables, with Bartlett’s test determining the sphericity of the sample data. Repeated measures ANOVA (2 × 3) was applied for intra and intergroup comparisons with the adjusted Bonferroni post-hoc test. The effect size was calculated to analyse the clinical impact of the interventions, as suggested by Cohen (Lakens, 2013), with < 0.2: weak; 0.2 to 0.79: moderate; > 0.8: strong. The significance level was set at p < 0.05. All the analyses were conducted using the SPSS program, version 23.0 (IBM, I.C.).
RESULTS
No significant intergroup difference was observed in any of the baseline measures. Repeated measures ANOVA (2 × 3) showed Wilk’s Lambda= 0.49; F= 3.70; p= 0.011; statistical power= 88% for the interaction between group and moment. Repeated measures ANOVA (2 × 3) showed a F= 4.54; p= 0.043 for CRP, F= 5.29; p= 0.010 for IL-6 and F= 1.95; p= 0.175 for IL-10.
In intragroup comparison, there was no significant change in CRP values over time for both groups compared to the baseline moment. However, the effect size (ES) indicated a moderate increase post and 24 h after maximal stretching compared to baseline. On the other hand, in the SG, the effect size demonstrated a moderate decline in CRP concentrations between pre and 24 h post-exercise. Intergroup comparison showed that post-exercise CRP was significantly higher in the MG compared to the SG (p= 0.035; Δ= 94%). Based on the ES, CRP levels were higher (strong effect) in the MG than the SG at all moments (pre, d= -1.97; post, d= 2.13; 24 h, d= 1.90).
Regarding IL-6 values, there was a significant increase 24 h after maximal stretching (p= 0.008; Δ= 44.16%) compared to baseline. The ES indicated increases of strong magnitude post and 24 h after stretching in the MG, and moderate (24 h after vs pre) and strong (pos vs 24 h after vs pos) increments in the SG. Although there was no significant difference between groups, the ES revealed higher values in the MG vs SG, with moderate (pre and pos) (pre, d= -0.21; post, d= 0.73) and strong (24 h after) (24 h, d= 1.06) magnitude of differences.
There were no significant intra and intergroup differences in IL-10 values. The data of inflammatory markers are presented in Table 3.
Table 3 Changes in inflammatory markers assessed pre-training, immediately after and 24 hours after training for each group*.
| Measurement times | Effect size (d) | ||||||
|---|---|---|---|---|---|---|---|
| Pre | Post | 24 h | Pre-post | Pre-24h | Post-24 | ||
| PCR (mg/mL) | SG | 0.54± 0.20 | 0.50± 0.22 | 0.46± 0.21 | -0.19 | -0.39 | -0.17 |
| MG | 0.73± 0.48 | 0.97± 0.76† | 0.86± 0.72** | 0.50 | 0.28 | -0.14 | |
| IL-6 (pg/mL) | SG | 2.59± 0.88 | 2.43± 0.65 | 2.99± 0.44 | -0.19 | 0.45 | 0.87 |
| MG | 2.40± 0.47 | 2.91± 120 | 3.46± 1.37# | 1.09 | 2.24 | 0.45 | |
| IL-10 (pg/mL) | SG | 5.11± 0.40 | 5.04± 0.13 | 5.09± 0.35 | -0.18 | -0.04 | 0.43 |
| MG | 5.41± 155 | 5.31± 1.18 | 5.39± 1.47 | -0.06 | -0.01 | 0.07 | |
*Data presented as mean± SD; SG: Submaximal Stretching group; MG: Maximal Stretching group;
**p< 0.05; Post vs. 24h-post; #p< 0.05; Pre vs. 24h-post; †MG Post vs SG Post.
DISCUSSION
The aim of this study was to investigate the effects of a flexibility session with two different intensities on the inflammatory responses in young, trained military personnel. An important point in this study was to assess the effect of flexibility intensity on different inflammatory markers, given that multiple cytokines should be measured simultaneously in order to evaluate inflammatory responses and better monitor the performance, recovery, and health of individuals (Lee et al., 2017).
Our results revealed that post-exercise CRP values were significantly higher in the MG when compared to the SG. The CRP values increased moderately post-exercise when compared to the basal MG values and remained moderately higher 24 h after maximal stretching in relation to the pre-exercise moment according to ES. Concerning IL-6, there was a significant rise 24 h after when compared to pre-exercise in the MG. These findings partially support the experimental hypothesis that intervention with maximal stretching would promote greater changes in CRP and IL-6 values after maximum stretching compared to submaximal stretching but without changes in IL-10.
This inflammatory response may be explained by the magnitude of force applied to muscles during high-intensity stretching (Apostolopoulos et al., 2015a; da Silva et al., 2021). Applying an overload induces transitory microdamage in the skeletal muscle system, resulting in an acute inflammatory response orchestrated by inflammatory markers derived from the affected tissues (Apostolopoulos, 2018; Pedersen, 2019; Da Silva et al., 2021). The extent of the inflammatory response is determined by the magnitude of muscle damage, inflammation, and injury-specific interaction between the invading inflammatory cells and the injured muscle (Peake, Neubauer, Della Gatta, & Nosaka, 2017; Da Silva et al., 2021).
The results of the present study corroborate earlier investigations, which reported increases in high sensitive PCR (hs-CRP) post and 24 h after high-intensity stretching when compared to less intense protocols (Apostolopoulos et al., 2015a) and the control (Apostolopoulos et al., 2015b). However, the above-mentioned studies applied flexibility protocols with greater volume, which may be associated with a late increase in CRP, but this did not occur in our study. Although isolated flexibility exercises were applied, scientific evidence suggests that long-duration static flexibility exercises (≥ 60 seconds per muscle group) showed greater performance deficits and substantial declines in muscle power and strength (Behm, Blazevich, Kay, & McHugh, 2016; Chaabene, Behm, Negra, & Granacher, 2019).
From the standpoint of the proinflammatory process resulting from biomarker response in the acute phase to flexibility training, Apostolopoulos et al. (2015a) observed a marked rise in hsCRP post and 24 h-post with intense stretching applied at 90% range of motion (ROM) when compared to 30% ROM and 60% ROM, suggesting a possible association between high-intensity stretching and inflammation. In a more recent study, Apostolopoulos et al. (2018) investigated the effects of flexibility training intensity on recovery from a non-habitual eccentric exercise of the right knee extensors in thirty recreationally active men and found significantly higher CRP values 24 h after intense stretching when compared to less intense stretching. The inflammatory response pattern for the post-exercise CRP values found in the present study reveals a number of similarities with the aforementioned studies in terms of the marked increase in CRP immediately after exercise, which was compared between the different flexibility protocols.
Regarding the effect of flexibility training on the other inflammatory markers, IL-6 increased significantly 24 h after maximal stretching, possibly triggering a proinflammatory environment. However, there was no significant rise in IL-10 at any of the assessment times. Increases in IL-6 levels associated with higher post-exercise CRP suggest that maximal stretching was responsible for the inflammation (Apostolopoulos et al., 2015b).
Despite not specifically addressing flexibility, we observed conflicting evidence when we compared our findings with those in the literature on inflammatory responses to different exercise intensities. Pozzolo et al. (2020) found no differences in the acute effect of aerobic exercise at different intensities (65 to 70% and 80 to 85% of estimated heart rate) on pre- and post-exercise IL-6 and IL-10 concentrations in apparently healthy university students, despite inducing different inflammatory responses. Uchida et al. (2009) found no changes in IL-6 concentration 24 h after compared to pre-exercise when assessing inflammatory responses at four different supine exercise intensities (50% of one-repetition maximum 1RM, 75, 90, and 110%), maintaining total workload, in military personnel.
On the other hand, Ghafourian, Ashtary-Larky, Chinipardaz, Eskandary, and Mehavaran (2016) observed a transitory inflammatory reaction, with significant increases in IL-6 and IL-6/IL-10, immediately and 24 hours after intermittent treadmill running at 85% of VO2max when compared to a single submaximal session (65% of VO2max). These different results may be due to the fact that, in addition to the intensity factor and physical exercises, the extent of the inflammatory response is determined by the degree of muscle damage, magnitude of the inflammation and injury-specific interaction between muscle and the invading inflammatory cells (Peake et al., 2017).
According to Peake et al. (2017), peak IL-6 serum level is reached at the end of exercise or shortly afterwards, followed by a rapid decline, then returning to basal levels. Thus, the combination between modality, intensity, and duration of the physical activity determines the magnitude of exercise-induced plasma IL-6 concentration. In the present study, we observed significant increases in IL-6 24 h after an intense flexibility exercise session but were unable to measure IL-6 values over longer times, precluding establishing a greater timeline or moments that could signal a possible return to basal values.
Thus, it is believed that physical exercise can modulate the immunological system via a local response, characterised by increased IL-6 expression in the active muscle, which subsequently stimulates the rise in systemic IL-10 levels (Neves et al., 2014). However, the IL-10 values obtained here did not trigger a sufficient anti-inflammatory reaction to release IL-10 after the application of different flexibility methods.
Our findings corroborate those reported by Cabral-Santos et al. (2019), who found no significant correlation between exercise intensity and changes in IL-10 when assessing the response of this marker immediately after an acute physical exercise session in healthy adults. However, our data conflict with those obtained by Cerqueira, Marinho, Neiva and Lourenço (2020), who reported an increase in IL-6 levels, followed by a rise in IL-10, which was more pronounced after intense exercise sessions. In the present study, IL-10 concentrations did not follow the increased IL-6 levels observed up to 24 h after exercise. Another factor that may explain this situation is related to exercise duration, given that the protocols used here applied a small amount of flexibility training. According to Cabral-Santos et al. (2019), exercise duration can be considered the most important factor in determining the magnitude of the increase in exercise-induced plasma IL-10.
A recent study concluded that high-intensity exercise causes acute inflammation followed by pain and performance impairment, confirmed by changes in post-exercise biochemical and immunological markers. However, after fifteen days, the values returned to baseline levels, suggesting that new overloads could be applied at the end of this process (Da Silva et al., 2021). In this sense, although acute inflammation is a transient inflammatory response and beneficial to the body, a persistent inflammatory response is associated with tissue dysfunction and pathology (Scheffer & Latini, 2020).
Thus, exercise practitioners, athletes, and coaches need to be aware of flexibility exercise intensity to avoid possible acute inflammation that could compromise (or impair) the application of new overloads. This consideration allows the monitoring of adaptive responses for an adequate balance between applied overload and recovery, preventing injuries and avoiding non-functional overreaching (Antunes Neto, Almeida, & Campos, 2017).
The limitations of the present study include difficult-to-control factors such as diet one week before and during the experimental procedure, emotional stress, and sleep of the study participants, in addition to the non-blinding of subjects and the researchers who applied the training protocols. However, the examiners were blinded to the laboratory analysis findings.
CONCLUSIONS
In summary, maximal stretching promoted increases in the inflammatory markers CRP and IL-6, with no changes in IL-10, in young-trained military personnel. As such, this method was more efficient in triggering a proinflammatory environment when compared to submaximal stretching as a function of having used greater exercise intensity. From a practical standpoint, our results suggest that maximal stretching should be prescribed with caution, given that its inflammatory potential may intensify muscle damage, affect acute recovery, and compromise performance. Moreover, we highlight the importance of distinguishing the submaximal and maximal stretching due to their specific impact on inflammatory profile.
Future research analysing a more diverse set of biomarkers of muscle damage and inflammation over a longer time course could help us understand the causes of inflammation and the time needed for muscle recovery when a new overload could be applied without muscle damage.