INTRODUCTION
Physical inactivity is a worldwide problem considered a global pandemic that is a risk factor for cardiometabolic diseases and is highly associated with mortality and comorbidities (World Health Organization, 2009). Worldwide, one in four young adults (28% or 1.4 billion people) are physically inactive (PI) (World Health Organization, 2018), while in Chile, in 2018, it was estimated that 57.4% of young adults were (PI), where, unfortunately, females have been reported to be less active (71.5%) when compared to males (54.7%) (Ministerio del Deporte, 2019). In this line, the older adults (i.e., population≥ 60 years) show a high prevalence of PI, comprising a 70.9% of prevalence, but worryingly this value is increased to 74.7% when the population age has more than 70 years (Ministerio del Deporte, 2019). Thus, despite the vast and corroborated benefits that physical activity promotes overall health in young and older adults (Reimers, Knapp, & Reimers, 2012), there is a high prevalence of PI in Chile. Additionally, due to ageing, there is higher comorbidities presence, such as poor nutrition (i.e., overnutrition/or underweight), poor glucose control (i.e., hyperglycemia or type 2 diabetes mellitus), and high blood pressure (i.e., prehypertension or arterial hypertension). Considering that there is also a tendency to be more PI after 46 years (Alley, Duncan, Schoeppe, Rebar, & Vandelanotte, 2017), there is a need to increase public strategies for promoting physical activity during ageing.
Physical activity has a beneficial role in the body composition in older adults, promoting increases in skeletal muscle mass (SMM), muscle strength, and decreasing body fat (Concha-Cisternas et al., 2019). For example, it was reported that higher and lower adiposity levels were inversely associated with significant cognitive impairment in older adults, making physical activity a regulatory factor to both SMM and adipose tissue (Concha-Cisternas et al., 2019). Epidemiological evidence has also shown that after 40 years, there is an accelerated decrease in SMM, significant muscle strength loss, and increased adiposity (JafariNasabian, Inglis, Reilly, Kelly, & Ilich, 2017). Thus, active ageing (i.e., adhering to the international physical activity guidelines of ≥ 150 min/week) promotes a lower cardiometabolic risk, a lower frailty syndrome, a better functional capacity, low depression levels, Alzheimer's disease, and finally, low mortality rate (Cunningham, O'Sullivan, Caserotti, & Tully, 2020). The international recommendations for physical activity for older adults promote moderate-to-vigorous activities, including exercises for muscle strength, flexibility, balance, and agility (Bull et al., 2020).
There are multiple benefits of resistance training that can aid in greater muscle activation for stair climbing, greater leg flexion/extension capacity, and thus significant capacity in the lower extremity muscles, which play a critical role in older adults in maintaining independence (Marshall, Morgan, Martinez-Valdes, & Breen, 2020). For example, it has been shown that older adults have low muscle activation in leg extensors after walking for 15 minutes (Marshall et al., 2020). However, it is also clear that part of the age discrepancies between older and young adults was shortened when older females walked for 30 minutes at the same intensity as young peers. Older females elicited a similar energy expenditure (Jones, Waters, & Legge, 2009). Other studies suggest that PI in older adults shows fewer steps per week than physically active (PA) peers (Ayabe, Ishii, Takayama, Aoki, & Tanaka, 2010). In this line, the muscle strength capacity directly relates to the jump performance, yet unknown in which population groups the PI condition promotes more or less detrimental effects.
On the other hand, although it is well established that under PI condition, young adults show higher levels of SMM, muscle strength, and lower adiposity levels than older peers (Amaral, Amaral, Monteiro, Vasconcellos, & Portela, 2019), unfortunately, there is yet unclear how PI impact to both young and older adults about health-related outcomes such as body composition, and SMM performance as muscle strength and jump performance. Thus, the present study aimed to describe and compare the body composition (i.e., SMM and body fat), handgrip strength, and countermovement jump (CMJ) between young and older females. We hypothesised that older females show a lower SMM and strength performance under the PI condition than young peers.
METHODS
A descriptive-comparative study with a quantitative approach and cross-sectional design, which evaluated 101 females selected through an intentional non-probabilistic sampling being participants distributed into four groups: (i) older physically active females (Old-PA; n= 26); (ii) older physically inactive females (Old-PI; n= 25); (iii) young physically active females (You-PA; n= 25) and; (iv) young physically inactive females (You-PI; n= 25).
The inclusion criteria were; (i) female sex; (ii) between ≥ 18 and 60 or more years; (iii) functionally independent in their basic activities of daily living, measured through the Barthel Index (Mahoney & Barthel, 1965); (iv) without cardiovascular, respiratory or musculoskeletal pathologies that can limit the participation in physical activity tests. The exclusion criteria were: (i) to have musculoskeletal injuries or to undergo physical rehabilitation treatment that impeded their typical physical performance; (ii) those who presented functional limitations to walk normally; (iii) those who had vertigo or balance disorders. The study was conducted in the Pedro Aguirre Cerda Gymnasium of Osorno City. In the afternoon session, in the first stage of the study, 150 females were recruited. Forty-nine females were excluded, 25 did not meet the inclusion criteria, and 24 did not attend all the measurement sessions (15 due to employee reasons and 9 due to stationary health reasons). Finally, 101 females were participants in the study (Figure 1). All participants were informed of the aim and procedures of the research and signed an informed consent authorising the data for scientific purposes. The research protocol was reviewed and approved by the Scientific Ethics Committee of the Universidad Autónoma de Chile (No. 06-2016), and the principles of the Declaration of Helsinki were followed for its development.
Physical activity levels measurements
The participants (young and older females) who do not adhere to the physical activity recommendations (≥ 150 min/week of moderate physical activity or ≥ 75 min/week of vigorous physical activity) according to the international physical activity questionnaire (IPAQ Research Committee, 2005), were categorised as PI, older (Old-PI), or young (You-PI) female. On the other hand, all these PA participants were older PA (Old-PA) and young PA (You-PA) females.
Anthropometry and body composition measurements
Bipedal height was measured using the Frankfort plane in a horizontal position, using a stadiometer (Bodymeter 206, SECA, Germany; with accuracy: 0.1 cm) fixed to the wall. Body weight and body composition were measured using a bioimpedance meter (InBody120, tetrapolar 8-point tactile electrodes system, model BPM040S12F07, Biospace, Inc., USA; with accuracy: 0.1 kg), with which SMM and body fat were determined according to previous recommendations (Marfell-Jones, Stewart, & De Ridder, 2012). This measurement was carried out in the morning with the participants fasting, without metallic objects and light clothing. Also, body weight was divided by bipedal height squared (kg/m2) to determine body mass index (BMI).
Handgrip strength
The test was applied following previous recommendations (Hernández-Martínez et al., 2018). A sitting posture was determined as the most appropriate for the assessment, including aligned spine, shoulders adducted and without rotation, elbow flexed 90° to one side of the body, forearm, and wrist in a neutral position. A handgrip dynamometer was used (Jamar®, PLUS +, Sammons Preston, Patterson Medical, Illinois, USA); its role was determined according to the hand's size, allowing a comfortable and functional grip of the instrument with an adequate closure of the joints. Phalangeal and interphalangeal metacarpus in a fist position, favouring contact between the index finger's first phalanx and thumb. Three attempts were made with the dominant and non-dominant hand, using the mean of the records’ maximum value. A two-minute rest interval was considered between each attempt.
Relative strength
The data of maximum handgrip strength divided by body weight (maximum total handgrip strength kg/body weight kg) were used (Porter, 2019).
Countermovement jump
The CMJ assessed vertical jump performance; all tests were performed on a mobile contact mat (Ergojump; Globus, Codogne, Italy). The woman was instructed to step onto the platform with her arms flexed and hands resting on her hips; after the signal, she was asked to descend and flex her legs by 90°, and on the evaluator's signal, the woman was to perform the jump. Following previous recommendations (Chi, Huang, Kernozek, & Hsieh, 2013), take-off and landing were standardised to full knee and ankle extension in the same place. The test was performed three times, separated by a two-minute rest interval. The maximum height of the three trials was adopted and expressed in centimetres. An evaluator was on each side of the contact platform to assist the participants in any eventuality in the test's execution.
Statistical analysis
Values were reported as mean± standard deviation. The Shapiro-Wilk test was used to determine the normality of the data, while the Levene test was used to assess the homogeneity of variance. Normal distribution was observed in anthropometry, body composition, handgrip strength, relative strength, and CMJ. The one-way ANOVA test with Tukey HSD post-hoc compared the Old-PA, Old-PI, You-PA, and You-PI groups. The α level was established at p< .05 for statistical significance, and the Cohen (1992) index test was used to calculate the effect size (ES). All calculations were performed with the STATISTICA statistical package (Version 8.0; StatSoft Inc., Tulsa, OK, USA).
RESULTS
Body weight did not show significant differences between the Old-PI and You-PI (p= .99; ES= 0.46), nor between the Old-PA concerning the You-PA (p= .94; ES= 0.49). Neither were significant differences found when comparing Old-PI with You-PA (p= .4; ES= 0.48), Old-PA with You-PI (p= .85; ES= 0.50) and You-PI with You-PA (p= .58; ES= 0.49).
The Old-PI presented a bipedal height significantly shorter than the You-PI (p= .04; ES= 0.20), Old-PA compared to the You-PA (p< .01; ES= 0.39), Old-PI with the You-PA (p< .01; ES= 0.76) and You-PI with the You-PA (p= .02; ES= 0.45). However, no significant differences were found between Old-PA and You-PI (p= .61; ES= 0.56).
The Old-PA presented a significantly higher BMI compared to the You-PA (p< .01; ES= 0.48), as did the Old-PI respect to the You-PA (p< .01; ES= 0.47), without finding significant differences between Old-PA and You-PI (p= .11; ES= 0.51), You-PI and You-PA (p= .10; ES= 0.49) and Old-PI with the You-PI (p= .62; ES= 0.52). The results as the mean and standard deviation are presented in Table 1.
Table 1 Characteristics of the sample.
| Participants (n= 101) | Old-PA (n= 26) | You-PA (n= 25) | Old-PI (n= 25) | You-PI (n= 25) |
|---|---|---|---|---|
| Age (years) | 70.5± 7.3* | 20± 2.1* | 72.2± 6.8* | 21.9± 2.5* |
| Body weight (kg) | 70.5± 13.8 | 72.5± 10.9 | 66.8± 13.0 | 67.6± 10.4 |
| Bipedal height (m) | 1.50± 5.0* | 1.72± 9.0* | 1.43± 30.9* | 1.56± 7.0* |
| BMI (kg/m2) | 31.7± 7.2* | 24.2± 2.5* | 30.4± 4.7* | 28.1± 5.1 |
BMI: body mass index; Old-PA: older female physically active; Old-PI: older female physically inactive; You-PA: young female physically active; You-PI: young female physically inactive. Values expressed as mean ± standard deviation;
*statistical significant difference p< .05.
A significantly higher SMM was presented in the Old-PI when compared with the You-PI (p< .01; ES= 0.57), You-PA compared to the You-PI (p< .01; ES= 0.56), Old-PA with the Old-PI (p< .01; ES= 0.46) and You-PA with the Old-PA (p< .01; ES= 0.53). No significant differences were found between Old-PA and You-PI (p= .07; ES= 0.53).
The You-PA showed significantly lower body fat compared to the You-PI (p< .01; ES= 0.44) and the Old-PA with the Old-PI (p< .01; ES= 0.43). On the other hand, the Old-PI reported significantly higher body fat than the You-PA (p< .01; ES= 0.35) and the Old-PA compared to the You-PA (p= .002; ES= 0.46). There were no significant differences between Old-PI and You-PI (p= .06; ES= 0.43) and between Old-PA and You-PI (p= .06; ES= 0.52).
The You-PA had a significantly higher handgrip strength than the Old-PA (p< .001; ES= 0.43), You-PI (p< .01; ES= 0.40) and Old-PI (p< .01; ES= 0.38). The Old-PA showed a significantly higher handgrip strength compared to the Old-PI (p< .01; ES= 0.45). No significant differences were found between Old-PA with You-PI (p= .16; ES= 0.46) and Old-PI with You-PI (p= .18; ES= 0.51).
The Old-PA presented a significantly higher relative strength compared to the Old-PI (p< .01; ES= 0.53), the You-PA compared to the You-PI (p< .01; ES= 0.41) and the You-PA with the Old-PI (p< .01; ES= 0.51). Without significant differences between Old-PA and You-PI (p= 0.73; ES= 0.52).
The Old-PA presented a significantly lower height in the CMJ when compared with the You-PA (p< .001; ES= 0.61), Old-PI concerning the You-PI (p< .001; ES= 0.65), and Old-PI with the You-PA (p< .01; ES= 0.26). The Old-PA obtained a significantly higher height in the CMJ with to the Old-PI (p< .01; ES= 0.35), You-PA compared with the You-PI (p< .01; ES= 0.63) and Old-PA with the You-PI (p< .01; ES= 0.51). The results as the mean and standard deviation are presented in Table 2 and Figure 2.
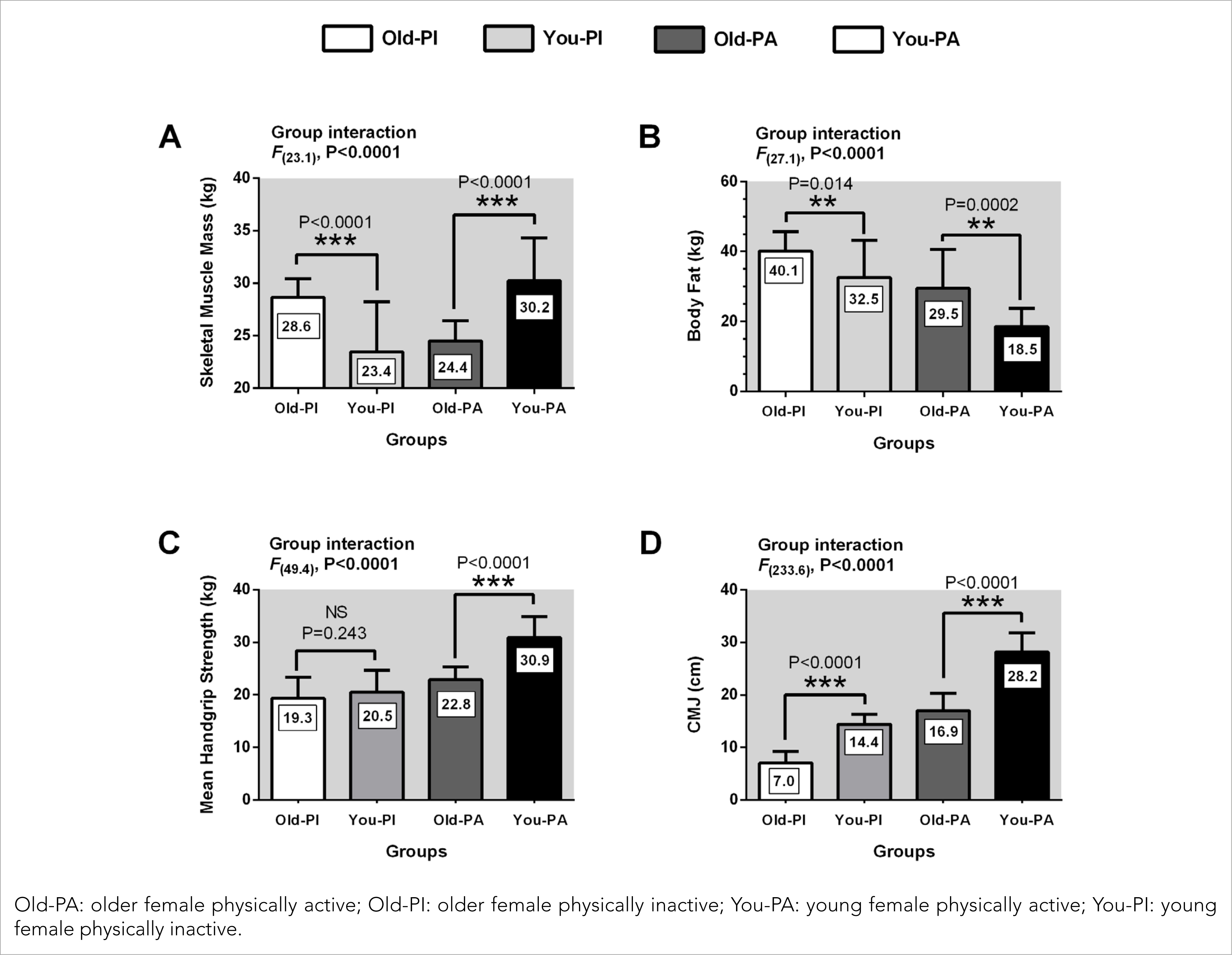
Figure 2 Comparison of body composition, handgrip strength and jump performance between physically active and physically inactive females.
Table 2 Comparison of body composition, handgrip strength, relative strength and jump performance between physically active and physically inactive females.
| Variables | Old-PA (n= 26) | You-PA (n= 25) | Old-PI (n= 25) | You-PI (n= 25) |
|---|---|---|---|---|
| Skeletal Muscle Mass (kg) | 28.6± 1.7* | 30.2± 4.2* | 24.4± 1.9* | 23.4± 4.9* |
| Body Fat (kg) | 29.5± 11.0* | 18.5± 5.7 | 18.5± 5.7 | 32.5± 11.7* |
| Handgrip Strength (kg) | 22.8± 2.4* | 30.9± 4.1* | 30.9± 4.1* | 20.5± 4.6* |
| Relative Strength (kg) | 0.33± 0.06* | 0.42± 0.06* | 0.42± 0.06* | 0.30± 0.09* |
| CMJ (cm) | 16.9± 3.3* | 28.2± 3.9* | 28.2± 3.9* | 14.4± 1.9* |
Old-PA: older female physically active; Old-PI: older female physically inactive; You-PA: young female physically active; You-PI: young female physically inactive. Values expressed as mean ± standard deviation;
*statistical significant difference p< .05.
DISCUSSION
The present study aimed to describe and compare body composition (SMM and body fat), handgrip strength, and CMJ between young and older females. The main findings indicate that: (i) under the condition of PI, CMJ (jump performance) is the main variable that suffers impairment in older females; (ii) despite being young, the situation of PI expresses a lower SMM in young females compared to PI older females; (iii) PA older females have decreased CMJ as well as lower SMM compared to PA young females; (iv) there are no significant differences in body fat and handgrip strength between PA older females compared to PI young females.
On the other hand, the Old-PA achieved higher values of SMM and CMJ than You-PI. This response is an encouraging fact due to the ageing process is also related to a decrease in SMM (Larsson et al., 2019) and sarcopenia (Steffl et al., 2017), while regular PA adherence has been shown to help maintain and improve SMM in older adults (Nishiguchi et al., 2014). In addition, maintaining the SMM stimuli through physical activity in old age increases the functional independence to perform the necessary activities of daily living (Steffl et al., 2017; Valdés-Badilla, Gutiérrez-García, Pérez-Gutiérrez, Vargas-Vitoria, & López-Fuenzalida, 2019). On the other hand, during ageing, changes in muscle fibres’ composition are identified, with a decrease in SMM (i.e. muscle fibres) at volume and number, especially in those of classification II of muscle fibres (i.e., fast twitch fibres), with an additional increase in adipose tissue at SMM (Choi, 2016). In this line, during ageing, there is a progressive decrease in SMM and muscle strength, significantly impacting functional performance (Choi, 2016), and observing the results of our present findings, independently of being an older or young female, the inactive physical state promote detrimental effects at this tissue. With ageing, SMM loss is usually accompanied by increased adipose tissue, both subcutaneously and viscerally (Carter, Justice, & Thompson, 2019). Increasing body fat reduces physical fitness, the immune response and increases hospitalisations (Maffetone & Laursen, 2020). Furthermore, the redistribution of body fat can occur in the SMM itself when the SMM's oxidative capacity is exceeded (Carter et al., 2019). Therefore, increased body fat concomitantly with SMM reduction can negatively affect jump performance, strength, and, thus, functional capacity (Nelke, Dziewas, Minnerup, Meuth, & Ruck, 2019). However, another point recently discussed in the literature refers to the decrease in muscle function due to intra and intermuscular fat infiltration and not specifically due to muscle atrophy, with the consequent reduction in the quality of SMM (Carter et al., 2019). The loss of muscle quality is called a deficit of SMM function instead of sarcopenia (Carter et al., 2019).
Our study shows that physical inactive harms SMM, even in a young female with an average of 22 years. A young female would be expected to have better levels of SMM than a PA older female regardless of physical activity participation since both SMM and muscle strength increase up to 30 years (Keller & Engelhardt, 2013). On the other hand, physical inactivity has been described as a risk factor for mortality, even more significant than overweight (McAuley et al., 2012). Maintaining adequate physical fitness becomes a powerful protective health agent (Barry et al., 2014). In this sense, performing physical exercise routines that stimulate muscle strength in older females becomes necessary since positive changes have been reported in inflammation, apoptosis, regeneration, and function of both muscle fibres and muscle anatomy (Steffl et al., 2017). By contrast, during ageing, there is an increase in body fat at the muscular, visceral, and liver level (Choi, 2016), a fact that is associated with a higher risk of obesity and sarcopenia in the aged, in addition to cardiovascular diseases, type 2 diabetes mellitus, metabolic syndrome, urinary incontinence and depression, elements that together affect functional independence (Amarya, Singh, & Sabharwal, 2014). On the other hand, a young female's increase in body fat negatively affects their physical fitness (Farhat, Iannotti, & Simons-Morton, 2010), which can reduce the health-related quality of life and general health in this age group (Mondal & Mishra, 2017). Also, regular physical activity practice produces a decrease in body fat in older females (Rossi et al., 2017), a situation that coincides with the results obtained in our study, where Old-PA showed lower body fat compared to Old-PI and You-PI.
During ageing, there is a decrease in handgrip strength (Hernández-Martínez et al., 2018); however, this phenomenon has been associated with sarcopenia, impaired mobility, frailty, and higher cardiovascular comorbidities, more than the aging processes (Lera et al., 2018). Higher handgrip strength levels, for example, represent better physical function and less deterioration in functional mobility (Pratama & Setiati, 2018). Relevant antecedents, since the Old-PA showed higher levels of handgrip strength than the Old-PI and You-PI in the present study.
On the other hand, an earlier decrease in muscle power (3% per year) compared to muscle strength (2% per year) has been reported in people over 70 years, healthy and with reduced mobility (Reid et al., 2014). Gains in lower body muscle power in older adults can lead to lower fall risk and better performance in functional tasks of daily living (Sayers & Gibson, 2014). Besides, a female who manifests a PA lifestyle from youth to old age preserves bone mass and performs better in jump performance, positively impacting the health-related quality of life (Xu, Lombardi, Jiao, & Banfi, 2016). Antecedents that support the results observed in the present study where the Old-PA showed greater height in the CMJ than the Old-PI and You-PI.
Among the main strengths of this study are: (i) the differences in the age and physical activity habits of the female analysed, which strengthens the evidence regarding the benefits of physical activity in old age (Reis et al., 2016; Steffl et al., 2017; Valdés-Badilla et al., 2019); (ii) the validity of measurements that are widely used in the scientific literature (Chi et al., 2013; Lera et al., 2018), favouring their application in other groups of the population. As limitations, we can point out: (i) the non-probabilistic selection of the sample that restricts the external validity of the results and; (ii) not controlling diet and sleep, which could influence the body composition and muscle performance of the female evaluated, although they were asked to maintain usually their eating and sleeping habits. Despite its limitations, this study provides contextualised information on the benefits of leading PA lifestyles.
CONCLUSION
The physically inactive condition is related to decreased CMJ in older females, where physically active peers have greater relative strength and jump performance. On the other hand, young physically inactive females have less SMM than older physically inactive females, a finding that requires further research.