Introduction
The desmoplastic fibroblastoma (DF) was first described by Evans in 1995 in a study of seven clinical cases.1 The designation “collagenous fibroma” was proposed by Nielsen et al.2 as a reference to the benign histological characteristics of the lesion. DF is a rare benign neoplasia originating from fibroblastic and myofibroblastic cells, but its etiology is still uncertain. Cytogenetic studies suggested a non-random association of translocation t(2;11)(q31;q12) with the DF, suggesting that this region harbors a gene of pathogenetic importance, causing, for example, a deregulated expression of FOSL1.3,4
Clinically, the lesions present as a firm mass of slow growth rate, not associated with pain, and commonly found in subcutaneous and intramuscular tissues of the arms, posterior region of the neck, and upper part of the back, rarely affecting the head or mouth.1,2,5,6 The disease is more likely to happen in male patients who are in the fifth and sixth decades of life but can affect patients of any age.7 Intraorally, DF presents clinically as a firm lesion that grows slowly, affecting sites like the palate, gingiva, and tongue. Histopathologically, it is composed of stellate-to-fusiform fibroblasts and myofibroblasts amid dense collagenization.8
The involvement of the oral cavity is extremely rare, and to the extent of our knowledge, there are only 14 cases reported in the literature so far.8-21 Therefore, this study details a new case in the alveolar ridge and compares its histopathological and immunohistochemical characteristics with the reports available in the literature concerning the DF diagnosis.
Case report
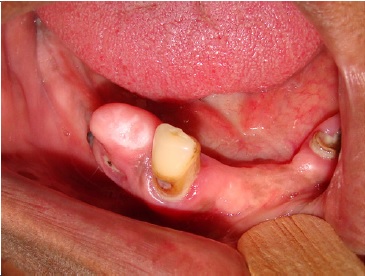
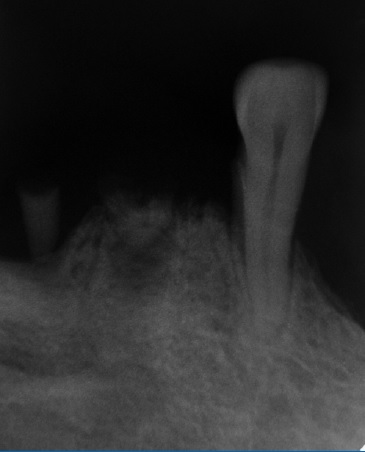
A 59-year-old woman was referred to the clinics of oral medicine of the Federal University of Ceará - Sobral Campus to evaluate an elevated lesion on the mandibular ridge that was painful during mastication. Intraoral inspection revealed a well-defined nodule measuring 10 mm in diameter, smooth-surfaced, and covered by healthy mucosa with whitish areas in the region of tooth 44 (Figure 1). The tumor was located in the edentulous region of the alveolar ridge without related trauma since the patient did not use a superior or inferior prosthesis. Close to dental remnants of tooth 43, the periapical radiography showed residual roots of teeth 44 and 45 associated with bone rarefaction (Figure 2). The patient reported having undergone a previous extraction in the lesion site but did not know when it had been performed.
Considering the clinical hypothesis of benign mesenchymal neoplasia and the size of the lesion, an excisional biopsy was performed. The specimen was fixed in 10% neutral formalin buffer and embedded in paraffin. Macroscopic examination revealed a soft-tissue fragment of whitish color, fibrous consistency, and irregular shape and surface, showing a homogeneous and whitish surface to the cut. The microscopic exam showed non-encapsulated proliferation of spindle-to-stellate fibroblasts in highly collagenous fibrous connective tissue.
Blood vessels were inconspicuous and generally sparse. Inflammatory cells were absent, and no mitotic figures or necrosis were present (Figures 3 and 4). Immunohistochemical reactions were performed with the streptavidin method. Neoplastic cells were strongly and diffusely positive for vimentin (Figure 5), and some cells were focally positive for alpha-smooth muscle actin (α-SMA) (Figure 6), CD99 (Figure 7), and β-catenin (Figure 8). The cells were negative for desmin, S-100, CD34, and ALK. The histological findings combined with the immunohistochemical profile are consistent with DF. The patient is undergoing dental follow-up and has been free from the lesion for 2 years.
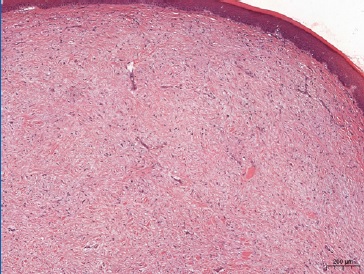
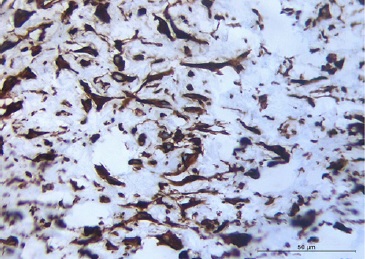
Figure 3 Histopathological features demonstrating a non‑encapsulated proliferation of spindle‑to‑stellate fibroblasts in highly collagenous fibrous connective tissue (hematoxylin and eosin staining, 40x magnification).

Figure 4 Approximated view showing some fibroblasts with large or oval nuclei. Conjunctive tissue with blood vessels of small diameter exhibiting perivascular fibrosis and fusiform cells of variable morphologies, large or oval nuclei (hematoxylin and eosin staining, 200x magnification).
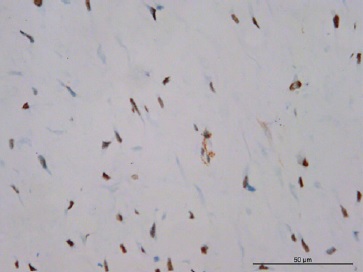
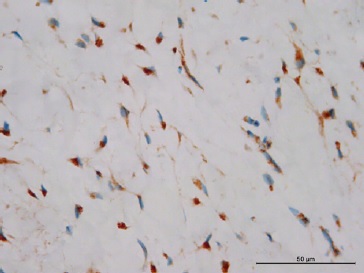
Figure 6 Positive expression of α‑SMA exhibiting myofibroblasts with nuclear staining (400x magnification).
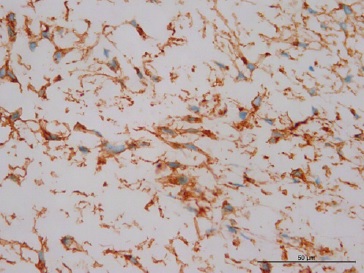
Figure 7 Positive expression of CD99 and staining of the cytoplasm of the fibroblasts (400x magnification).
Discussion
The case described in this report has clinical, pathological, and immunohistochemical characteristics coincident with other DFs that affected the oral cavity.8-21 However, the anatomical location of this case coincides with only one8 of the 14 cases reported in the literature and has different symptomatology from most of them.
The DF may arise despite no identification of traumatic injury or inciting event.9,17,18,21 We have not identified any traumatizing agents or other irritating factors that could justify the tissue proliferation. The absence of a triggering event or a specific cause of reactive fibrous proliferation corroborates the hypothesis that this lesion is actually a neoplastic process.8 Although we found bone rarefaction in the same region of the DF in the imaging exam, it was not associated with the tumor but with the presence of dental remnants. Alveolar bone loss has not been described in any case of oral DF. Furthermore, other reports of oral DF have shown that even though the lesions grew close to potentially irritating factors such as dental calculus, periodontal disease, teeth with extensive caries cavities, and removable prostheses,8,20,21that did not necessarily mean they were in contact with or caused by those factors.
In the literature, patients affected by oral DF were mostly females (four women for each man) and had a mean age of 52 years old. As Table 1 shows, only one case was diagnosed in a child. Clinically, according to the literature, the DF is a mass of tissue of firm or elastic consistency and a slow growth rate.8-21 Moreover, the lesions are well-circumscribed, the color might be similar to the oral mucosa, red, yellowish, or purplish, and the size can vary from 7 mm to 6 cm.8-21 Our patient was a 59-year-old woman whose tumor had clinical characteristics similar to the ones described in the literature. Interestingly, this type of pathology is not associated with pain, but its presence can interfere with speech, chewing, and closing movements of the mouth.15 In this case, the patient did not know the injury’s onset time but sought dental care due to pain caused by it. We believe that the lesion caused that pain during mastication.
Table 1 Clinical features and immunohistochemical profile of the published cases of oral desmoplastic fibroblastoma.
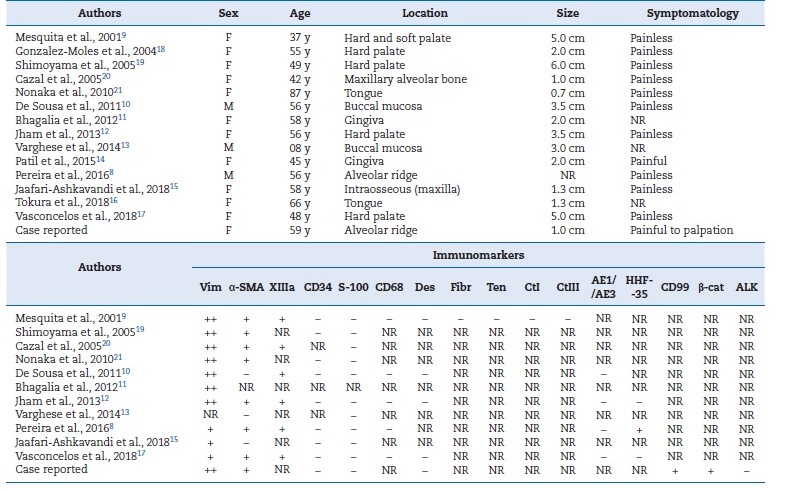
Vim: vimentin; α‑SMA: α‑smooth muscle actin; XIIIa: XIIIa factor; Des: desmin; Fibr: fibronectin; Ten: Tenascin; CtI: collagen type I; CtIII: collagen type III; β‑cat: β‑catenin; F: female; M: male; y: years; ++: diffuse positive in tumor cells; +: focal positive in tumor cells; -: negative in tumor cells; NR: not reported.
Lesions that are painless or non-damaging to oral function are often neglected by patients, leading to delayed diagnoses. The differential diagnosis of DF includes a variety of soft-tissue lesions such as fibrous inflammatory hyperplasia, traumatic fibroma, giant cell fibroma, neurofibroma, schwannoma, myofibroma, lipoma, nodular fasciitis, fibromatosis, and pleomorphic adenoma.9,18 Jham et al.20 reported a case of a pedunculated DF on the hard palate and suggested the giant cell fibroma, pyogenic granuloma, and leaf-like denture fibroma as differential diagnoses of DF with the non-fixed base.
Histologically, the DF is a paucicellular tumor of fusiform pathological cells with oval nuclei and small nucleolus.5 Binuclear and multinuclear giant cells can be observed in the conjunctive tissue, which can exhibit scarce blood vessels and the absence of inflammatory cells such as lymphocytes, plasma cells, and histiocytes. Adipocytes and skeletal muscle might also be observed, and focal calcification in the center of the tumor was seen in only one of the cases reported.8-21 Thus, the histological differential diagnosis of DF should include inflammatory fibrous hyperplasia (IFH), giant cell fibroma (GCF), traumatic fibroma (TF), benign mesenchymal neoplasms such as myofibroma, neurofibroma, and solitary fibrous tumor (SFT), and less frequent soft-tissue lesions like nodular fasciitis and fibromatosis.8,9,15,18 Myofibroma differs from DF because it usually presents a biphasic pattern consisting of fusiform cell fascicles alternating with areas with a hemangiopericytoma-like form.22
Fibromatosis is composed of a proliferation of well-differentiated fibroblasts and myofibroblasts within a collagenous- to-myxoid stroma, but with an infiltrative growth and is more cellular than DF.8,23 Although SFT presents thick bands of collagen, there is a patternless architecture of hypercellular and hypocellular areas separated from each other by this hyalinized collagen not found in DF.24 Fibroblasts with stellate morphology or binucleated are rarely found in IFH, GCF, and TF, but these lesions, particularly IFH and GCF, do show a greater level of cellularity and contain associated inflammatory infiltrate.5,8
In the present case, a proliferation of spindle-to-stellate cells was observed without an inflammatory infiltrate. Nakamura et al.25 demonstrated that oral irritation fibromas with spindle cell proliferation and absence of inflammation showed no expression of α-SMA, which may suggest the absence of myofibroblasts. Moreover, clinically, oral irritation fibromas have small diameters, commonly located on the buccal mucosa along the occlusion line, on the labial mucosa, and on the tongue, affecting the gingiva in a few cases.25 These features differ from most of the reports of oral DF, in which α-SMA immunopositivity is observed,8,9,13,15-17,20with the tumors ranging from 0.7 cm to 6 cm in diameter (mean 2.7 cm) and located on the buccal mucosa less frequently.8-21 Previous reports of DF show adjacent fat and skeletal muscle in a histopathological examination.5,9 However, this was not observed in this case because the alveolar ridge is free of fat or skeletal muscle.
Although the immunohistochemical evaluation is interesting for an accurate DF diagnosis, it is not mandatory.18 The immunohistochemical profile of DF reveals a predominantly fibroblastic origin with focal myofibroblastic differentiation (Table 1).8 In the DF, tumor cells are negative to S-100 protein,8,9,11,13,15-18,20,21CD68,8,9,13,18,20desmin,9,13,18,20AE1/AE3,8,13,18,20and CD34, which have had positive expression only on endothelial cells,8,9,11,13,15,17,18,20as observed in this case. Diffuse positivity for vimentin,8,9,11,13,15-20 focal immunopositivity for factor XIIIa,8,9,13,16,18,20and α-smooth muscle actin8,9,13,15-17,20 has been observed, similar to the present case. Mesquita et al.9 used other immunomarkers such as fibronectin, tenascin, and type I and III collagen, but all of them had a negative expression on the tumor cells of DF lesions.
The immunohistochemical analysis is an important diagnostic tool in cases of oral DF where the histopathological examination is not enough to differentiate it from other diseases. Similar to the DF, the SFT tends not to be immunoreactive for the S-100 protein; however, it is also not for α-SMA, and is characteristically immunoreactive for CD34, contrary to DF.24 Myofibroma shows a panel very similar to DF, characterized by positivity with vimentin and α-SMA and negativity for S-100 protein, desmin, CD68, and CD34, but its architectural pattern visualized under hematoxylin and eosin staining differs from DF.22 Fibromatosis, similar to DF, tends to be positive for vimentin and negative for CD34 and S-100 but differs from DF due to its desmin positivity.23 The GCF is negative for α-SMA, unlike DF.8
The case reported in this investigation is the second to identify a DF on the mandibular ridge and the second where painful symptomatology was registered. Therefore, we offer a review of the important immunohistochemical markers that will guide oral pathologists to conclude cases of difficult histopathological diagnosis.