Introduction
Plastic in the medical sector was valued worldwide at 18.9 billion euros in 2019.1,2 In Europe, this market is expected to reach a value of 4 billion euros by 2024 due to its growing demand.3 The World Health Organization (WHO) explains that 85% of the health sector waste is non-infectious. However, only a small percentage is recycled, while most end up in landfills (79%) or incinerated (12%). Consequently, the medical sector represents around 4% of global greenhouse gas emissions - if it were a country, it would be the fifth most polluting country in the world.4,5
According to the Eco Dentistry Association (EDA), some 680 million plastic and paper protections and 1.7 billion sterilization instruments and packaging are sent to landfills or disposed of in the environment per year.6-8This situation raises another problem: overexposure to bisphenol A (BPA) and di(2-ethylhexyl)phthalate (DEHP) and microplastics (small particles between 100 nm and 5 mm). Recent studies show that large amounts of microplastics end up in the human diet and have been found, for example, in seafood, honey, bottled water, and alcohol, as a result of their deterioration in the environment.3-5,9 In our body, due to the inability of our immune system to eliminate plastic, this situation can lead to chronic inflammation and cancer.10 Also, the contact between microplastics and antibiotics in our body has adverse effects. In fact, the correlation between antimicrobial resistance and increasing
plastic pollution is under investigation. The findings indicate that when in contact with antibiotics, plastics can promote genetic mutations in bacteria that cause them to acquire antibiotic resistance, creating possible threats to human health.11,12
Reducing plastic consumption at hospitals and medical/ dental offices is a difficult task since medical areas have benefited most from its use.13-16Plastic is economical, heat resistant, long-lasting, versatile, biocompatible, requires less energy to be produced compared to metal or glass, and offers a sterile environment.1,13 These assets that make plastic the ideal material for single use also make it almost impossible for nature to eliminate.10,17 In addition, single-use plastics currently represent 85% of all plastics in health.13 Because recycling these plastics in the health sector carries risks of cross-infection and contamination, the current destruction methods aim to provide the population with more security.1,8,15-18
The recently published study “Environmental sustainability practices in Portuguese dental clinics” concluded that clinical directors showed good environmental awareness and satisfactory implementation of environmental sustainability practices in dental clinics and that costs were the most reportreved barrier to implementing further practices. This study triggered the interest in finding a safe, easy, cheap solution for dental clinics to implement that would also be sustainable and eco-friendly, allowing the mitigation of the effects of the waste produced by single-use plastic.17,18
For the reasons stated above, sterilization pouches were considered the ideal specimen for this study because they do not come into contact with the patient and consist of a paper/plastic bag with one side made of medical-grade paper and the other made of a thin plastic film. They cover medical instruments before their sterilization in the autoclave and are currently thrown away after the sterilized instruments are unpacked.
The problem addressed is not only the usage of plastic but also how it is discarded. One of the complications related to reusing these plastics is that they are marked as single-use by manufacturers.19
In the impossibility of replacing plastic with other materials because of its many assets, this study aims to question the safety and efficacy of reusing sterilization pouches without compromising their sterilization conditions.20,21 Therefore, the question and hypothesis raised are: Can sterilization pouches be used a second time while maintaining their sterilization conditions?
Material and methods
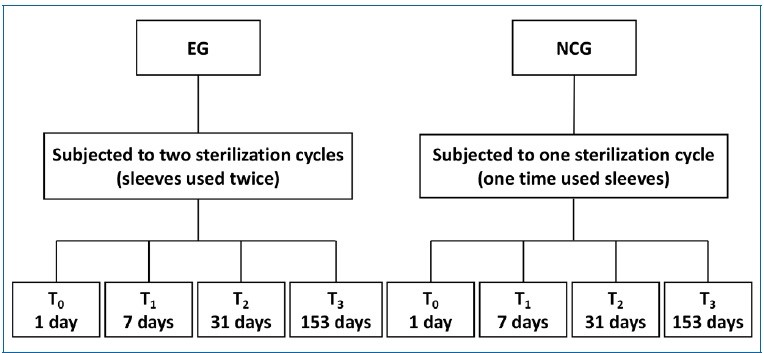
Paper/plastic pouches were prepared from non-sterile sterilization rolls (MEDISTOCK®, France), with 7.5x15.0 cm each, and randomly selected to undergo a sterilization cycle in na autoclave at 121°C and 15 psi for 33 minutes (HS-22 K5+ WHITE, GENTINGE®, Sweden) to mimic their use in dental practices and hospitals. After that cycle, pouches that met the inclusion criteria were selected as the Experimental Group (EG) and opened to undergo a second sterilization cycle (n=12). The inclusion criteria were not presenting any openings, water drops inside, bends and creases, or burns.
The pouches that did not pass the quality check to be reused -exclusion criterium- were discarded. A 3 cm x 2 cm non-sterilized gauze dressing (Bastos Viegas®, S. A., Portugal) was placed into each of the once-used pouches: EG pouches; Negative Control Group (NCG) - once-used pouches; and Positive Control Group (PCG) - environmentally contaminated pouches. Afterward, the NCG and the PCG pouches were sealed at 1 cm from the base and 3 cm from the top with a thermal sealer (EuroSeal® 2001, Euronda S.p.A., Italy), and the EG pouches were resealed at 6 cm from the top (because they were being reused) with the same sealer. In this step, the exclusion criteria were again applied to ensure/maintain integrity.
All pouches were arranged in a horizontal position, with the paper side of one pouch in contact with the plastic side of the next one without touching the chamber wall of the autoclave. The specimens were sterilized at 121°C and 15 psi for 33 minutes.
After the sterilization cycle, specimens were stored in na opened plastic box in a microbiology laboratory (Laboratory of Microbiology Applied to Health, LMAS, University of Minho) to recreate an open microorganism-rich environment, for 1 day (T0), 7 days (T1), 31 days (T2), and 153 days (T3) at room temperature (≅ 20ºC) and humidity (≅ 40%). After each storage period, the pouches were inspected for barrier damage before being opened. Figure 1 shows the design of the study in a flow chart.
The gauze dressings were removed aseptically (MSC-Advantage™ Class II Biological Safety Cabinets, Thermo Fisher Scientific™, USA) and incubated (general incubator with builtin roller or shaker - NB-205Q, N-BIOTEK, South Korea) in nutriente agar (Research Products International - RPI, USA) in Petri dishes at 37°C for 3 days. After incubation, the Petri dishes were inspected, and microbial contamination was assessed and classified as present or absent based on the visible changes in color and shape of the mediums.22 The results were compared regarding the different periods of storage and sample groups.
Lastly, the sample size determination was carried out in Microsoft Excel Spreadsheet Software, taking into consideration the following study objectives: evaluate the presence of contamination in each of the three groups (EG, NCG, and PCG) at four different times (T0, T1, T2, and T3); compare the contamination levels between the two groups at each moment; and compare the contamination levels between the four moments in each group.
Results
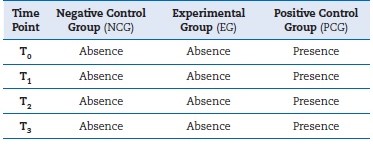
All pouches tested passed the reusability inspection, and none of the samples was discarded based on the exclusion criteria. Table 1 presents the results obtained after assessing the nutrient-agar Petri dishes containing the gauze dressings from the groups assayed (EG, NCG, and PCG) and for the diferente sample storage periods. As observed in Figure 2, the EG specimens showed no sign of contamination (≅ 0% microbial contamination), even after 5 months of the experiment, similar to the NCG (≅ 0% microbial contamination) and contrary to the PCG (≅ 100% microbial contamination).

Figure 2 Microbiological result of the experimental group (EG), negative control group (NCG), and positive control group (PCG), respectively.
The PCG was intentionally contaminated by exposure to a normal microbiology laboratory environment -the same environment where EG and NCG specimens were stored- and showed extensive microbial growth in all samples after the tested period. On the contrary, both EG and NCG specimens remained sterile over the storage time.
Discussion
This preliminary exploratory study focused on evaluating the reuse of sterilization paper/plastic pouches commonly used as packaging material in the healthcare sector. The results from this study show that sterilization pouches can be used a second time while maintaining sterility and integrity compared to once-used sterilization pouches, even for extended periods (153 days - 5 months of storage).
Avoiding cross-infection is a high priority in the healthcare sector for patients’ safety, and, evidently, there should be no financial or material barriers to preventing the risk of healthcare-acquired infection.5,6 However, nowadays, healthcare waste is causing significant environmental contamination from single-use plastics, which in turn, harms human health.11-14 Knowing that infection control is critical, this study wanted to provide a solution that would be safe and environmentally friendly.
In the studied conditions, once-used pouches (NCG) and twice-used pouches (EG) kept sterility and integrity conditions for 5 months, showing that they can be reused at least once.
The PCG samples presented microbial contamination after the whole storage period, reinforcing the storage conditions in a microbiologically contained environment. It should be noted that all sample groups were stored in the same conditions and, thus, would be prone to contamination if the pouches were compromised in terms of integrity and sterility.
The results of the present work are in line with recent investigations presented by Puangsa-Ard et al.19 and Klumdeth et al..23 In both studies, no microbial contamination was found in either the twice-used or the once-used pouches, and all remained sterile for up to 6 months. However, those studies’ settings differ from the present ones. This study was designed and conducted to recreate the daily environment of a dental practice, considering the opinion of researchers that proper
storage is crucial to maintain sterility and that the storage environment conditions are a more relevant factor than the type of packaging material.24,25 Thus, sterilization pouches were stored in an environment similar to a clinical practice where samples were more susceptible to microbial contamination.
Moreover, due to the possibility of damage imperceptible to the human eye, the pouches used in this study were subjected to some manipulation, making them more prone to event-related contamination. However, the material used in this assay-gauze-is less prone to compromise their reuse, unlike sharp and rough materials. These findings indicated that reusing paper/plastic sterilization pouches could be a great start on this adjustment to a more sustainable and environmental practice.
It should be emphasized that pouches must meet minimal criteria to undergo a second sterilization cycle, which requires special care. Thus, reusing paper/plastic pouches implies special handling/procedures and careful, thorough reusability inspection by healthcare workers to guarantee their sterility and reusability.
Future investigations should repeat the experiment and do other more robust microbiological tests. Because this was a preliminary experimental scientific research aimed at providing a solution that would be safe in every procedure performed in healthcare facilities, all colony-forming units visible on the Petri dishes were classified as contamination, regardless of the number of colonies. It would also be appropriate to use two types of medium and/or broth or specific/selective culture media for double-checking contamination in the future,26 use a larger sample size, and place medical or dental instruments inside the sterilization pouches. Further research should also determine the breaking point of how many times these sterilization pouches can be reused and how long they can preserve the sterile environment.