Introduction
Coronal restoration of teeth following root canal treatment generally requires additional support from the root canal system, which endodontic post systems can provide.1 Fiber-reinforced posts have a significant proportion of fibers inside a polymer matrix that constructs highly cross-linked structures.2 Recent studies2,3showed that post systems should have equal or similar rigidity to the tooth to transmit occlusal forces equally on root surfaces. Nowadays, fiber-reinforced posts used with advanced adhesive systems are a great restorative option because of their high aesthetic properties, flexibility, and similar elasticity to dentin.3
Adequate bonding among the fiber post, dentin, and adhesive cement is important and provides increased restoration stability.4 The bonding effectiveness depends on the smear layer (SL) removal and the adhesive system.5 After preparing the fiber post, the SL covers the dentin tubules, restraining the bonding of adhesive cements and sealers.6 The SL consists of organic and inorganic particles and bacterial products, which promote infection persistence and decreased intratubular dentin penetration.7
Thus, the SL removal enhances the penetration of sealers int dentin tubules and facilitates root canal cleaning.8 Various chemical substances can be used to modify the hybrid layer’s longevity and decrease its breakdown, with collagen cross-linkers, protease inhibitors, and enzyme inhibitors being the primary agents. These techniques are well described in the literature.9,10 The elements of the targeted SL dictate these medications’ modes of action. Deproteinizing compounds can be used to break down the organic componentes of the SL, while chelating agents can bind to the inorganic components.11,12 Furthermore, SL removal can be enhanced by hand-adjusting the application of adhesive cement.13
Current methods of SL removal depend on chemical irrigation, irrigation activation techniques (IATs), and their combination.14 Some IATs, such as manual, sonic, ultrasonic, and laser-activated techniques, get irrigation solutions in contact with the whole root canal system for effective action.15 When using a needle for traditional irrigation, only the tip of the needle is in contact, which can lead to less disinfection activity.7 Sonic irrigation (SI) produces mechanical oscillation largely at the tip of the file. In contrast, passive ultrasonic irrigation (PUI) systems produce acoustic waves and combine them with the chemical action of the irrigation along the noncutting file.16 This microstreaming action moves the irrigation agente along the surface of the canals and enhances mechanical cleaning.17 Some studies have shown that activation with sonic and ultrasonic systems provides cleaner surfaces than traditional irrigation with a needle.18,19
Laser-activated irrigation (LAI) is an IAT using medium-infrared lasers (2780 and 2940 nm) that has emerged as a supplementary technique for irrigating root canals. Water-based solutions strongly absorb the radiation emitted by the laser, causing the formation of inflated and collapsed vapor bubbles at the fiber tip - a process known as cavitation. Variations in collapsed bubble dimensions result in localized shock waves and different fluid motions. Consequently, further cavitation bubbles are induced by succeeding laser pulses. Thus, the irrigant creates acoustic streaming that flows throughout the entire root canal system.15 Er:YAG and Er,Cr:YSGG lasers show great potential as methods for stimulating irrigants. According to Aldeen et al.,20 using Er:YAG laser resulted in a much higher debris removal than PUI and traditional irrigation.
PIPS (photon-induced photoacoustic streaming) is an LAI technique that uses a pulsed Er:YAG laser. This technique employs low pulse energies (10 or 20 mJ) and a short pulse length (50 μs), which leads to high peak powers and effective cavitation.21 This method differs from previous laser IATs by inserting only the tip into the pulp chamber, thus avoiding any contact with the root canal wall. It has been shown to produce a mass of photoacoustic shockwaves capable of spreading the irrigation solutions into dentinal tubules, removing the SL on the dentinal walls.22 Since the development of lasers, PIPS has been used to disinfect the root canal space and remove the SL and other foreign matters.23 PIPS is one of the emerging LAI techniques since 2012. It has lower energy and short pulse length compared to the other LAI methods, thus decreasing thermal damage to the root canals and periodontal tissues.24 Shock wave-enhanced emission photoacoustic streaming (SWEEPS) was developed to enhance the debridement efficiency of the PIPS method.25 When the cavitation bubble starts to collapse, a secondary pulse is transmitted through the liquid, causing the formation of a second cavitation bubble. This second cavitation bubble speeds up the collapse of the first one, resulting in a powerful collapse and finally releasing a shock wave.26 However, there is very little data about SL removal with LAI systems compared to ultrasonic and sonic activation systems.27,28
The aim of this in vitro study was to compare the diferente IATs (sonic, ultrasonic, and laser-activated) for their ability to remove SL after post-space preparation. The null hypothesis tested was that there are no differences in efficiency between the IATs used in this study in removing the SL after the postspace preparation.
Material and methods
According to the ANOVA test estimation, with a 0.05 margin of error and a 0.7 power, the minimum number of samples for the study was 36 in total. Since the nonparametric test would be used, 10% was added, and the total number of samples became n = 40.29
This study’s sample consisted of 40 mandibular premolars with a single root. These teeth were extracted due to orthodontic or periodontal issues and had not undergone root canal treatment. Teeth of comparable lengths were selected. Afterward, periapical radiographs were obtained to verify the presence of single canals in the teeth. Teeth with excessively oval anatomy and more than one root canal were excluded.
Teeth with fractures and resorption, as assessed with a stereomicroscope, were also excluded. Residual periodontal tissues and calculus were removed from root surfaces with curettes, and the teeth were preserved in a sterile saline solution until their use.
After measuring working lengths using a stereomicroscope (Zeiss Stemi, Jena, Germany), the teeth were cut to a standardized length of 15 mm. Apical patency was verified using a #10 K-file. Premolars were prepared with ProTaper Next X1, X2, and X3 (Dentsply, Ballaigues, Switzerland), and root canals were irrigated between each file with 2 mL of 2.5% NaOCl using a 30 G side-vented needle attached to a plastic syringe (Canal Clean; Biodent, Paju, South Korea) placed 1-mm short of the working length. Before filling, a final irrigation with 2 mL of 2.5% NaOCl, 2 mL of 17% EDTA, and 2 mL of 2% chlorhexidine, alternated with a saline solution rinse between irrigants, was performed. Subsequently, the teeth were dried using sterile paper points (ProTaper Next X3; Dentsply Sirona, Ballaigues, Switzerland) and filled by the single cone technique using X3 (tip 30, 0.04 taper), gutta-percha (Dentsply, Ballaigues, Switzerland), and an epoxy-resin root canal sealer (Diadent Dia Proseal, Seoul, Korea). After the root canal obturation, access cavities were closed with Teflon tape and Cavit (3M Cavit, Minnesota, USA). Then, the teeth were immersed in distilled water to let the resin sealer (Diadent Dia Proseal, Seoul, Korea) polymerize, keeping the teeth in a moist environment without bacterial contamination. A week later, a 10-mm post-space preparation was done using 1.2-mm and 1.4-mm fiber post drills (Cytec, Hahnenkratt, Germany). Between each drill, teeth were rinsed with 2 mL of saline solution.
After this procedure, the samples were randomly divided into four groups for the final irrigation with the corresponding IAT:
• Group 1 (control group, no activation): Final irrigation was done with a conventional needle (2.5 cc, 27 G) with 3 mL of 17% EDTA for 1 min. Then, the teeth were rinsed with a 5-mL sterile saline solution.
• Group 2 (SI group): Final irrigation activation was done with an SI system (Endo Activator, Dentsply, Santa Barbara, USA) with 3 mL of 17% EDTA at 10,000 cycles per minute using a medium polymer tip (#25/.04) positioned at the post space and activated for 1 min. Then, the teeth were rinsed with a 5-mL sterile saline solution.
• Group 3 (PUI group): Final irrigation activation was done with a PUI system (Satelec Acteon, Merignac, France) with 3 mL of 17% EDTA for 1 min. The PUI system consisted of a noncutting #25 file (Irrisafe; Satelec Acteon, Merignac, France) driven by an ultrasonic device (Satalec P5 Newtron XS; Satelec Acteon, Merignac, France). Ultrasonic activation was done for 3 × 20 sec at 50% power for
1 min, with the tip immersed in the root canal containing irrigant throughout the post space. Then, the teeth were rinsed with a 5-mL sterile saline solution.
• Group 4 (LAI group): Final irrigation activation was done with an LAI device (Waterlase iPlus Biolase, Irvine, USA) with 3 mL of 17% EDTA for 1 min. The laser device was operated at a wavelength of 2780 nm, a panel setting of 0.5 W, and a frequency of 20 Hz, resulting in an energy output of 25 mJ per pulse. No air or water spray was employed during the procedure. The pulses were concentrated using a fiber tip (RFPT5) with a diameter of 580 μm. Then, the teeth were rinsed with a 5-mL sterile saline solution.
After the final irrigation procedures, the teeth were cut off buccolingually into two pieces. Then, all the teeth were dried and stabilized on the aluminum base. Teeth were covered twice with a 20-nm gold-aluminum layer and examined apically from the coronal reference under backscattered electron scanning microscopy (SEM) at 2000x magnification. Their SL removal was graded as follows:30 (0) all dentin tubules can be seen, there is no SL; (1) some of the dentin tubules are open, and some of the dentin tubules are covered with SL; (2) all dentin tubules are covered with SL. In previous studies,31,32 the area examined was described as the coronal, middle, or apical third of the post space, so we divided the post-space cavity into those three sections to compare the results with these previous studies.
The images obtained from the SEM were assessed, and two professionals with expertise in endodontics rated the SL removal.
The inter-operator reliability for these ratings was high, with a Pearson correlation coefficient of 0.994. Two independente and experienced examiners scored the SL removal, and if no consensus was reached, a third independent examiner was involved.
The Kruskal-Wallis H test was employed to compare the four groups, while the Mann-Whitney U test and Bonferroni correction were used for pairwise comparisons. The Friedman test was employed to compare the three post-space regions, while Wilcoxon’s signed rank test and Bonferroni correction were applied for pairwise comparisons. The statistical software SPSS 23.0 (SPSS, Chicago, IL, USA) was used, and a p-value below 5% was considered statistically significant.
Results
In this study, 40 mandibular premolars with post-space preparation were irrigated with four different IATs, whose efficiency in SL removal was evaluated under SEM (Figure 1). Table 1 details the mean and standard deviation values of SL removal scores for the used techniques. The PUI and control group performed superiorly in the coronal third compared to the middle and apical thirds. In turn, LAI was more successful in the middle and coronal thirds than in the apical third. SI achieved higher levels of success in the middle third of the post space compared to other regions. However, there was no statistically significant difference between the IATs (p>0.05). There was also no significant difference in SL removal for each IAT between the post-space apical, middle, and coronal thirds.
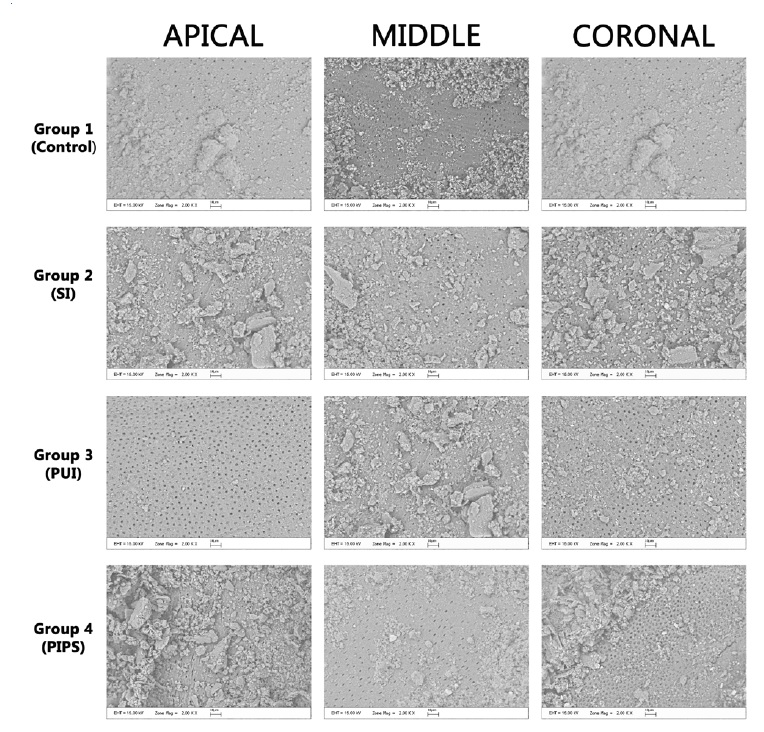
Figure 1 Representative electron scanning microscopy (SEM) images of the smear layer removal by irrigation protocols after post-space preparation.
Table 1 Mean and standard deviation values of smear layer removal scores in each irrigation activation technique group per post-space third.
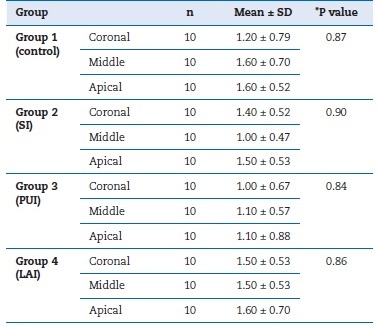
*P value indicated the difference among each group’s coronal, middle, and apical parts. SI - sonic irrigation; PUI - passive ultrasonic irrigation; LAI - laser-activated irrigation.
Discussion
The adoption of fiber post restorations has grown over time because of its great aesthetics and dentin-like elastic modulus.3 Adhesive cementation allows passively positioning the post system into the root canals.48 The success of adhesive bonding to dentin in endodontically treated teeth repaired by fiber posts and resin luting systems relies on the establishment of micromechanical retention through a demineralized surface and resin tag formation.33 Optimal adherence requires penetration into dentinal tubules, promoting a stronger bond between the tooth structure and the restorative material.34
The SL is an uneven layer developed during the mechanical preparation of post systems.14 In order to achieve successful dentinal adhesion, the SL should be removed either fully or partially.35 Previous studies8,36 recommended removing the SL to improve irrigants’ antibacterial activity, decrease the reinfection risk, and improve the penetration of sealers, adhesives, and intracanal medications. SL removal is accomplished by irrigants capable of dissolving organic and inorganic layers.14
Sodium hypochlorite (NaOCl) is a flexible antimicrobial solution frequently employed to destroy the biofilms of diferente microorganisms during endodontic treatments.37 Hard tissue deproteinization is another option.38 Research has shown that NaOCl modifies the material’s mechanical characteristics by dissolving the organic components of dentin and that 0.5% NaOCl is less effective on dentin deproteinization than concentrations of 1.0% and 2.25%. However, thorough research is lacking regarding the specific quantitative impacts of low doses (0.5-2.25%) and brief exposure periods (1-10 minutes) of NaOCl.39 Furthermore, the binding strength between dentin surfaces and fiber posts has been demonstrated to decrease after 10 minutes of exposure to 5.0% NaOCl.40
In turn, the prolonged use of EDTA in root canal treatments may contribute to root canal resorption because of its demineralizing impact on root dentin.41 EDTA strengthens the binding between endodontic sealers and dentin by exposing amino groups to demineralization aided by strong decalcifying agents.
Nonetheless, there is a significant danger of weakening the link and increasing interfacial deterioration42 due to the sealer’s inadequate penetration into the demineralized dentin, mostly derived from the severe decalcification of the root canal walls.
Furthermore, chelating chemicals like EDTA can modify additional dentin qualities, including micro and nanohardness.43 While removing the SL, irrigants must be in direct contact with canal walls for effective disinfection.7 Chow et al.44 reported that the efficacy of irrigation was related to needle depth. Munoz & Camacho-Cuadra45 showed that irrigants reached no more than 0-1.1 mm beyond the needle tip with conventional needle irrigation, offering decreased disinfection of the complete root canals. Moreover, conventional needle irrigation is less effective in reaching complicated anatomies like anastomoses, isthmus, and lateral canals.6 Shahravan et al.46 stated that removing the SL increases cleanliness and provides tight root canal filling. For this reason, different irrigant activation and delivery devices have been recommended to increase the distribution and effect of irrigants.32
Previous studies47,40 reported that IATs like sonic or ultrasonic may promote better disinfection efficacy by irrigants than conventional irrigation. However, Silva et al.49 reported no significant difference between conventional irrigation and PUI. PUI creates acoustic streaming and cavitation with noncutting action to remove the SL and disinfect the root canals.
Compared to ultrasonic energy, sonic activation has low-frequency vibration and flexible tips that do not deform canal walls as metal ultrasonic activation devices do.50,51 Capar & Arı Aydınbelge52 reported that PUI presented lower SL scores than SI. Conversely, Rödig et al.53 reported significantly greater SL removal with the SI method compared to PUI. In turn, Jensen et al.19 reported no significant difference in cleaning efficiency between SI and PUI techniques. In this study, there was no significant difference between PUI and SI techniques. This inconsistency can be explained by the varying thickness and amount of SL depending on the different root canal file systems and irrigation protocols used during the preparation. LAI eliminates excessive enlargement of the root canals, and placing the laser tip in the coronal portion of the pulp chamber generates the spreading of photoacoustic waves.15
Koch et al.54 stated that LAI with the PIPS technique increased fluid flow more than ultrasonic activation. Recent studies55,56 reported that using PIPS resulted in excellent debris and SL removal without thermal damage to the dentin surface. Di Vito et al.57 reported that EDTA activation with PIPS had more cleaning efficiency than conventional irrigation. Likewise, in Ayranci et al.’s study,58 the activation of NaOCl and EDTA by PIPS resulted in more successful SL removal than PUI with the same irrigants. Conversely, Akyuz et al.14 found no significant difference between PIPS and SI. Similarly, the present study found no statistically significant differences between PIPS, PUI, and SI activation techniques. Therefore, the null hypothesis was accepted. This finding may result from the different percentages, types, and amounts of irrigants used and the duration of the activation procedure. Further research is required to establish a standardized protocol for the activation time, irrigants, and type of activation.
This study had some limitations, such as SEM evaluation of only 2D constructed areas of the root canal space, including sclerotic dentin. The area of sclerotic dentin reduces SL removal by irrigants, which can affect the study’s outcome. In SEM imaging, similar areas of canal walls were evaluated, but obtaining images in the same areas is challenging. Also, it is difficult to measure the thickness of the residual SL with SEM imaging. Moreover, SL production depends on the instrumentation.
However, untouched root canal walls usually remain the same during preparation. A significant limitation of our study was its laboratory-based environment. It is crucial to note that the results may vary slightly if the same procedures are used on a living being. Based on the findings presented here, it is imperative to devise alternative protocols to better prepare the dentinal canal wall for adhesive resin cementation in endodontically treated teeth for future research.















