Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Obstétrica e Ginecológica Portuguesa
versão impressa ISSN 1646-5830
Acta Obstet Ginecol Port vol.10 no.3 Coimbra set. 2016
REVIEW ARTICLE/ARTIGO DE REVISÃO
Trends in the treatment of genital prolapse: a review of apical suspension techniques
Tratamento cirúrgico do prolapso urogenital: revisão das técnicas de suspensão apical
Inês Pereira*, Guillermo Willy Davila M**
Departamento de Obstetrícia, Ginecologia e Medicina da Reprodução - Centro Hospitalar Lisboa Norte, Lisboa, Portugal
Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Gynecology - Cleveland Clinic Florida, Weston, EUA
*Interna do Internato de Ginecologia e Obstetrícia do Hospital de Santa Maria
**Section of Urogynecology and Reconstructive Pelvic Surgery - Department of Gynecology, Cleveland Clinic Florida, Weston, FL, USA
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Genital prolapse is a highly prevalent disorder in women with negative impact in quality of life and sexual function. For uterovaginal prolapse, surgical repair may or may not include a hysterectomy, but the reestablishment of the vaginal apical support is always mandatory to ensure long-term effectiveness. Choosing the most appropriate surgical procedure should take into consideration many factors, such as the location of the anatomical defects, the severity of symptoms, the woman’s level of activity or concerns regarding the treatment’s durability. In this article we review data that can help to guide decisions when treating women with apical genital prolapse.
Keywords: Posthysterectomy vaginal vault prolapse; Uterine prolapse; Sacrospinous fixation; Sacrocolpopexy; Apical sling procedure
Introdução
Genital or pelvic organ prolapse (POP) is a highly prevalent pelvic floor disorder in the female population, adversely affecting women’s quality of life and sexual function.
Prolapse can be defined as a descent of the vaginal walls and/or uterus towards or through the vaginal opening, resulting from the protusion of pelvic organs from their normal anatomic positions. Pelvic support defects include multiple categories, which can occur in isolated or combined forms, including anterior vaginal wall prolapse, posterior vaginal wall prolapse, uterine prolapse or posthysterectomy vaginal vault prolapse.
Based on physical examination, the prevalence of POP varies between 30 and 40%1,2, and according to large epidemiologic studies, 6% to 8% of women report a sensation of a mass bulging into the vagina3,4.
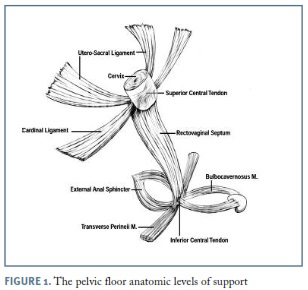
Despite its high prevalence, there is limited knowledge about POP pathophysiology. Prolapse may result from defective supportive structures submitted to normal levels of intra-abdominal pressure or from a normal pelvic support system subjected to chronically high levels of intra-abdominal pressure. Although loss of support can occur as a result of damage to any of the pelvic floor supportive structures, including the bony pelvis, the different components of the endopelvic fascia, the pelvic diaphragm or the perineal body, an increased interest in the role of levator ani muscle injuries has been developed over the last years5,6. (Figure 1)
Different cross-sectional epidemiologic studies have linked numerous factors to the risk of developing genital prolapse. Among them, demographic factors like age or menopausal status, obstetric factors like parity or number of vaginal deliveries, lifestyle factors like obesity, smoking or high-impact exercise, surgical factors like previous hysterectomy or POP repair surgery, and medical factors like chronic constipation or connective tissue disorders2,7,8.
Managing pelvic organ prolapse can be a challenge. Since there are multiple treatment options for symptomatic POP, including expectant management, conservative measures and surgery, decisions on treatment selection should be based on the magnitude of symptoms and their impact in women’s daily life activities.
The goal of prolapse surgery is not only to restore anatomy but also to maintain or improve function of all affected organs. Since several support defects often coexist, it is important for pelvic surgeons to acknowledge that single compartment corrections don’t always restore normal function to all the pelvic floor organs.
The role of apical compartment defects in combined POP has been addressed in different clinical studies, emphasizing why apical prolapse procedures can be critical to achieve the best outcome with vaginal prolapse surgery 9,10.
In this article we discuss established options and new trends in the surgical treatment of mid compartment defects, reviewing data that can help guide decisions whenever treating women with isolated apical and/or combined pelvic organ prolapse.
Initial evaluation
Accurate diagnosis is crucial for proper design of a comprehensive treatment plan, and obtaining a patient history is the key for understanding patient’s symptoms and expectations.
The diagnosis of POP lies on the combination of symptoms and pelvic examination findings. As most parous women, especially those of higher parity, usually show some degree of pelvic relaxation, it is crucial to understand if women who have genital prolapse are actually symptomatic.
Symptoms of POP typically include a vaginal bulge, pelvic pressure or heaviness, abnormal voiding or defecation and sexual dysfunction. Although there is no obvious anatomic threshold correlated to the presence of symptoms, the hymen appears to be an important landmark, as prolapse symptoms usually increase with descent through this anatomic level11,12.
Besides clarifying symptoms, it is also important to address how those symptoms interfere with the patient’s daily activities. Since prolapse treatment aims at improving quality of life (QOL), knowing exactly what issues are more bothersome for the patient can be of great help when deciding on a treatment plan or evaluating the outcome of an intervention. For that purpose several quality of life questionnaires have been developed and validated in different languages. The most commonly used include: the Pelvic Floor Distress Inventory (PFDI), the Pelvic Floor Impact Questionnaire (PFIQ), the Incontinence Severity Index (ISI) or the Urogenital Distress Inventory Form (UDI)13.
A careful pelvic examination with Valsalva maneuver is mandatory to establish specific site defects, as apical compartment defects frequently coexist with anterior and/or posterior vaginal wall prolapse.
Mapping and grading the prolapse using a validated tool such as the Pelvic Organ Prolapse Quantitative System (POP-Q) is needed, as well as evaluating vaginal mucosal status and the presence of urinary or fecal incontinence. According to the POP-Q system, uterine or vaginal vault prolapse can be identified by descent of point C with Valsalva maneuver (with C marking the cervix or vaginal cuff scar) from its normal position, which is usually at the total vaginal length minus 2 cm.
Preoperative urodynamic evaluation should also be considered in order to clarify bladder symptoms and exclude occult urinary incontinence.
Although asymptomatic women do not require treatment and may continue under observation, women who are symptomatic should initially be offered conservative interventions. Conservative measures may improve symptoms and withhold progression of the prolapse, but they will not be able to cure the underlying anatomical defects. The options include physical therapy to improve function and support to the pelvic floor structures, such as kegel exercises or biofeedback guided pelvic floor muscle training. Mechanical interventions, such as the placement of a vaginal pessary, are a type of treatment often offered to women with lower degrees of POP or to those unwilling or unfit for surgery. Pessaries require regular care by the patient herself or a clinician.
Selecting a surgical procedure
For women with prolapse requiring surgical treatment, one of the biggest challenges is to choose which surgery to perform, which route to use and whether a native-tissue or a graft-augmented repair is preferable.
If an apical support defect is noted on physical examination, it is crucial to include correction of the apex at the time of surgery, since it can be highly associated with the surgical repair durability and risk of recurrence10.
In addition to the anatomic location and severity of the prolapse, factors such as patient’s overall health and activity level, patient’s desire for uterine preservation or the presence of other concurrent pelvic floor symptoms, should all contribute to the decision of which surgery to perform.
Regarding the surgical treatment of apical defects, different approaches can be chosen and several reconstructive techniques have been described in the literature14. Surgery can be performed vaginally or abdominally, including (or not) a hysterectomy in the overall procedure, if the uterus is still in situ. Since there are benefits and risks to either approaches, surgical counseling requires extended knowledge on the type of procedures available, as well as the rates of prolapse recurrence and potential complications for each one of them.
Transvaginal procedures
Native-tissue reconstructive surgery
All transvaginal surgeries should be performed in a supine lithotomy position with the patient’s legs elevated in Allen stirrups and hips not flexed beyond 90° in order to prevent nerve compression injuries.
Which native-tissue support procedure to use depends on the surgeon’s training and expertise, since the available data comparing the various transvaginal native-tissue reconstructive repairs didn’t show significant differences among them in terms of efficacy or safety15,16. The concurrent use of vaginal hysterectomy, the shape and length of the vagina and the severity of the prolapse may also influence decision-making.
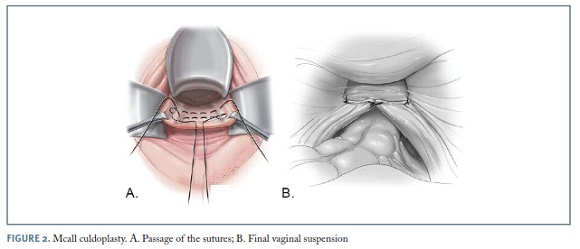
McCall culdoplasty
For uterovaginal prolapse, when there is no desire for uterine preservation, vaginal hysterectomy followed by a McCall culdoplasty and appropriate anterior and posterior vaginal wall repairs, can be an effective option to relieve symptoms, restore normal anatomy and function to the pelvic floor organs, and also to prevent future vaginal apical prolapse15. While suturing the uterosacral ligaments more proximally, the procedure also incorporates the proximal posterior vaginal wall. (Figure 2) McCall culdoplasty allows not only for the closure of the redundant cul-de-sac preventing potential bowel herniation, but also provides apical support and lengthening of the vagina.
The outcomes of this procedure were reviewed by Sze and Karram16; in an early study reporting 367 patients, 88% received postoperative follow-up from 1 to 12 years, reporting a cure rate of 88-93% and a recurrence rate of 11%. As for complications, the most worrisome risk reported is ureteral injury or kinking, occurring in approximately 2-4% of cases. For this reason, it is imperative to perform intraoperative cystoscopy after tying the suspension sutures in order to ensure ureteral patency17.
Being a simple, effective and safe procedure to perform, many specialists agree to consider McCall culdoplasty as part of every vaginal hysterectomy, even in the absence of prolapse, in order to minimize the risks of future mid-compartment defects.
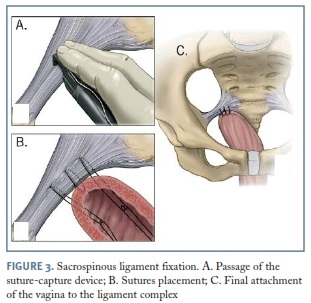
Sacrospinous ligament fixation
Sacrospinous ligament fixation (SSLF) is usually an option for moderate to severe posthysterectomy vaginal vault prolapse, although it can also be performed with a simultaneous vaginal hysterectomy or even as a form of hysteropexy, if there is a desire for uterine preservation. Usually this operation requires simultaneous correction of the anterior and posterior vaginal walls as well as an enterocele repair, if these defects are also present.
To safely and correctly perform this procedure, surgeons must have sustained knowledge of pelvic floor anatomy. Sacrospinous ligaments extend from the ischial spines on each side of the pelvis to the inferior portion of the sacrum and coccyx, and can be identified by palpating the ischial spine and tracing the triangular thickening towards the sacral bone.
For SSLF, delayed absorbable and/or permanent sutures are used to anchor the vaginal apex to the sacrospinous ligament, in an extraperitoneal approach. Reaching the ligament(s) for sutures placement can be achieved by an anterior or posterior vaginal approach, carefully dissecting the vaginal mucosa from the anterior or posterior wall in order to develop the vesicovaginal or the pararectal space, respectively. Although SSLF can be done bilaterally, the stitches are usually placed unilaterally to one side of the pelvis, resulting in a slight deviation of the vaginal axis laterally and downward to the sacrospinous ligament of choice17. (Figure 3) We prefer a bilateral approach for symmetrical support, although it can be related to a transitional increase in dyspareunia and obstipation.
While sacrospinous fixation has shown to be effective for vaginal apex support in early cohort studies, uterovaginal prolapse tends to recur with time, most commonly in the anterior compartment. In a study of 243 women, who underwent sacrospinous ligament fixation and vaginal repairs with a 73-months follow-up, prolapse recurrence rate in the anterior, posterior and apical segments were 37.4%, 13.6% and 8.2%, respectively18. The same study showed prolapse-free survival rates at 1, 5 and 10 years of 88.3%, 79.7% and 51.9%, respectively. Although recurrence rates may vary according to outcome definitions, they can reach up to 27% of women19.
Rare but serious intraoperative complications can occur with SSLF, and they include: hemorrhage from overzealous dissection involving the hipogastric venous plexus and the gluteal and pudendal vessels, which can be difficult to control; nerve injury and rectal perforation17.
High uterosacral ligament suspension
An alternative approach to correct apical prolapse is the bilateral high uterosacral ligament suspension (HUSLS). As a transperitoneal technique, it is often performed vaginally after a hysterectomy, although it can also be accomplished abdominally or by laparoscopy. Suspending the vaginal apex to the endopelvic fascia of the uterosacral ligaments, it directs the vaginal axis in the midline to the hollow of the sacrum, not creating any significant distortion of alignment, contrary to SSLF, and without involving any permanent mesh implant. Usually two to three delayed absorbable sutures are passed through the uterosacral ligament on each side of the pelvis and then through the full thickness of the anterior and posterior vaginal walls at the apex. After closing the vaginal cuff, tying the apical suspension sutures elevates the apex high up into its normal anatomical position. The major difference between the McCall culdoplasty and the HUSLS lies on the number of sutures and their level of placement along the uterosacral ligaments. While the first HUSLS suture is usually placed at the same level of the McCall culdoplasty, the second usually reaches the ligament higher in its midportion near the ischial spines, and the third is placed even higher, at the level of the sacrospinous ligament-coccygeus muscle complex, where a segment of the uterosacral ligament inserts.
According to published data, prolapse may recur in 25-30% of women who undergo HUSLS20,21. Margulies and colleagues showed that, in the anterior, apical and posterior vagina, the pooled rates for successful anatomic outcome were 81.2%, 98.3% and 87.4%, respectively, and that patients with more severe POP (stages III and IV) had significantly worse cure rates20. The most common complication reported after a HUSLS is sciatic-type pain, as a result of compression or entrapment of sacral nerve roots with the stitches, occurring in up to 7% of women22,23. Although ureteral injury should also be a concern, the actual risk is much lower then initially reported, staying at 1-2% in recent large series 20,21.
Obliterative surgery
Obliterative surgery corrects apical prolapse by removing and/or closing off all (colpectomy) or a portion of the vaginal canal (colpocleisis), reducing the pelvic organs present back into the pelvis. These procedures may be suitable for women with posthysterectomy vaginal vault prolapse or for women with uterovaginal prolapse who desire uterine preservation or in whom hysterectomy may be too risky16.
Obliterative procedures are less invasive and better tolerated by frail, older women, and are usually reserved for patients who are not candidates for more extensive surgery or do not plan future vaginal intercourse.
When performed in the appropriate population such procedures typically have shorter operative time, decreased perioperative morbidity, extremely low risk of prolapse recurrence and high patient satisfaction24-27. The obvious disadvantages lie in the elimination of the potential for vaginal intercourse, as well as the inability to evaluate the cervix or uterus via a vaginal route, the last in cases of uterovaginal prolapse when a Le Fort colpocleisis is performed.
All colpocleisis procedures remove vaginal ephitelium and then appose the anterior and posterior vaginal muscularis. By apposing the anterior and posterior vaginal walls the prolapsed apex becomes inverted and the sutured tissue forms a column of pelvic support. Whether performing a partial or total colpocleisis, 3 to 4cm of distal vaginal epithelium should be left in place to avoid placing traction on the posterior urethra when suturing the anterior and posterior vaginal muscularis28. In order to narrow the introitus and build up the perineum, a distal levator plication followed by and aggressive perineorrhaphy should always be performed as the final step of the procedure27.
Concomitant hysterectomy can be performed with an obliterative procedure, although case series data suggest that performing hysterectomy at the time of colpocleisis increases operative duration and morbidity 29,30. Nevertheless, hysterectomy may be advisable in women with risk factors for cervical or endometrial cancer even though there are no studies evaluating obliterative procedures in these sub-populations.
Transvaginal mesh surgery
Transvaginal mesh procedures were developed in an effort to combine the benefits of mesh-augmented repairs with a less invasive approach such as the vaginal route, in order to decrease the rate of recurrent prolapse seen with native tissue repairs and minimize potential surgical complications of intra-abdominal surgery.
Since the early 2000’s many different brands and types of prepackaged mesh kits were introduced in the market. The initial kits used long transcutaneously placed needles to attach mesh to the arcus tendinous fasciae pelvis or the sacrospinous ligaments, creating a hammock that supports the apex along with the anterior or posterior vaginal wall, depending on mesh placement.
Although total vaginal mesh procedures have shown to be superior to native tissue reconstructive surgery for apical and anterior wall repairs, with lower recurrence rates over time, new and unique complications related to the mesh itself and needle placements rose with time, creating a specific concern about the safety of these procedures31. Vaginal reconstruction can now be performed through the primary dissection incisions, using internal fixation fasteners and soft tissue anchoring systems or tissue fixation systems for internal anterior, apical and posterior repairs. The implementation of these techniques reduces the current avoidable technical problems of total mesh kits reducing the volume of mesh required for reconstruction and increasing the accuracy of anchoring to the supportive ligaments avoiding the blind passage of needles through long distances in the pelvis.
Mesh-related complications extend from functional complications, such as chronic pelvic pain, leg and groin pain, vaginal pain or dyspareunia, to anatomical distortions due to mesh exposure through the vaginal epithelium, erosion into adjacent organs or mesh contraction. According to a recent Cochrane Review on pelvic organ prolapse surgery, mesh exposure is one of the most common complications seen with total vaginal mesh procedures, with an overall exposure rate of 11.4% and at least half being symptomatic and requiring reoperation for treatment31.
In face of the potential hazards of total vaginal mesh surgery, the US Food and Drug Administration (FDA) has issue a Public Health Notice in 2008 and a Safety Communication in 2011 stating that “serious complications associated with surgical mesh for transvaginal repair of POP are not rare” and that “physicians should obtain specialized training for each mesh placement technique and be aware of the risks of surgical mesh”. As a result of these statements some mesh prolapse kits were voluntarily withdrawn from the market, with the current available prepackaged mesh kits being mainly to attach mesh to the sacrospinous ligaments.
Surgeons and researchers recognize that there may be a role for transvaginal mesh in POP surgery, given the risk of prolapse recurrence after native tissue repairs. New efforts in developing optimal materials and placement techniques for vaginal mesh procedures have taken place, in order to minimize the risk of mesh-related complications while maintaining a long—lasting surgical repair32. Recommendations regarding which patients may be appropriate candidates for mesh use have also been published33.
Biological graft materials are not associated with the material-related issues synthetic mesh involves, minimizing postoperative pain, general morbidity, mesh erosion or contraction34.
Abdominal procedures
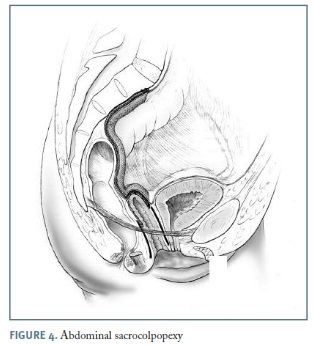
Abdominal sacrocolpopexy
Given the relatively high rates of prolapse recurrence with vaginal native-tissue reconstructive surgery, surgeons looked into other options that could afford more durability when correcting apical prolapse.
Abdominal sacrocolpopexy (ASC) is performed by securing the anterior and posterior vaginal walls, via a mesh bridge, to the anterior longitudinal sacral ligament overlying the sacral promontory, reestablishing a nearly horizontal axis to the vaginal canal 35. Grafts available for this procedure include synthetic materials like polypropylene mesh and biologic materials such as xenografts or allografts.
In the usual technique, a permanent mesh is attached to the posterior vaginal wall from the level of the rectal reflection and another piece to the anterior vaginal wall just above the bladder trigone, being acceptable to use a mesh fashioned into a Y shape or to use two separate strips of mesh. The mesh strips are then sutured to the sacral promontory anterior longitudinal ligament (Figure 4).
Although abdominal sacrocolpopexy (attachment between the sacral promontory and the vaginal vault in women who underwent total hysterectomy) is the most commonly performed procedure, uterine (sacral hysteropexy) or cervix (sacral cervicopexy) sparing procedures can also be performed.
When compared to native-tissue vaginal repairs, ASC has demonstrated more durability in multiple randomized trials31. In a systematic review of studies from 1996 to 2004, Nygaard and colleagues reported anatomic success rates after abdominal sacrocolpopexy ranging from 76 to 100% with a 4% reoperation rate for recurrent prolapse36. Nevertheless, these benefits should always be weighed against longer operating times, longer recovery and potential major complications. Being one of the major concerns of synthetic mesh-augmented surgery, erosion rates are estimated to range from 3.4% to 10.5% for ASC31,36,37. Erosion rates may vary depending on the type of mesh used, with type I polypropylene mesh showing the lower rate of erosion36.
Other potential complications of ASC are similar to those of major abdominal surgery, including hemorrhage, ureteral damage, bowel, bladder or rectum perforation and extrafascial wound infection.
Since the purpose of POP surgery is to improve quality of life, it is important to acknowledge that although ASC may be a more durable approach, it may also require repeat surgery for mesh issues in up to 5% to 10% of patients and although intraoperative complications are uncommon, they can be life threatening.
Minimally invasive sacrocolpopexy
Though classic sacrocolpopexy is typically performed through a lower abdominal incision, minimally invasive approaches such as conventional and robot-assisted laparoscopic sacrocolpopexy have gained popularity over the last decade.
Observational studies suggest that conventional laparoscopic and robot-assisted routes result in shorter hospital stays, faster recovery periods and less postoperative pain than abdominal sacrocolpopexy, with comparative short-term efficacy38-40. A important disadvantage to consider in these minimally invasive procedures is extended operative time, usually one to two hours longer, in addition to the higher costs involved, specially with robotic-assisted laparoscopy41.
Since performing a sacrocolpopexy requires suturing, some surgeons may prefer robotic-assisted laparoscopy in order to overcome technical limitations. Nevertheless, two randomized trials have found that robotic compared with conventional laparoscopic sacrocolpopexy has an even longer operative duration (24 to 67 minutes longer), with similar complication rates and short-term outcomes42,43.
Uterine preservation outcomes in uterovaginal prolapse
Traditionally all surgical repairs for uterine or uterovaginal prolapse begin by performing a vaginal hysterectomy, and for clinical purposes, uterine preservation is usually only considered whenever future fertility is in question. However, women are increasingly requesting uterine preservation for reasons other than fertility, including body image, sexuality or cultural preferences.
In a review on this topic, Ridgeway and colleagues concluded that uterine preservation might be a valid option in an appropriately selected group of patients44. Moreover, reassuring results with laparoscopic suture hysteropexy and sacrospinous hysteropexy, have also been reported45,46.
Preserving the uterus while addressing uterovaginal prolapse may have additional advantages over maintaining reproductive function. Operative morbidity and hospital stays are reduced compared to when hysterectomy is performed47,48, and uterine preservation is also related to lower rates of mesh erosion in procedures that involve the use of synthetic mesh49,50.
Though almost all the suspension techniques described in the previous sections can be performed as a hysteropexy procedure, some important modifications are necessary. Nevertheless, it is important to remember that uterine-sparing procedures are not appropriate in the set of cervical dysplasia, abnormal uterine bleeding or high risk for uterine malignancy, since evaluation and management of these problems may be more difficult to address after a utero-sparing prolapse repair.
Summary
Vaginal apical support contributes significantly to the reinforcement of all pelvic floor compartments. Thus, surgeons who perform surgical repairs for POP must be comfortable and proficient in choosing and executing the appropriate apical suspension for each particular patient. In women with advanced exteriorized POP, vault prolapse must be suspected until proven otherwise.
The standard use of McCall culdoplasty or uterosacral ligament suspension at the time of vaginal hysterectomy, in order to provide adequate support to the vaginal apex, is of paramount importance and decreases the incidence of late enterocele and posthysterectomy vaginal vault prolapse.
Transvaginal native-tissue apical repairs are probably the best option for older women who are sexually active, who have less severe prolapse or who have higher surgical risks. Older women with more severe degrees of POP, who are not interested in maintaining sexual function, may specifically benefit from an obliterative procedure. In younger active women and those with more severe or recurrent prolapse, sacrocolpopexy using a polypropylene graft, either by open or laparoscopic assisted routes, would be a first line option. Recently developed minimally invasive vaginal graft-augmented techniques have a role as an equivalent effective option for combined forms of POP involving the apex, however with concerns regarding mesh related complications.
REFERENCES
1. Samuelsson EC, Victor TF, Tibbin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol 1999, 180:299-305. [ Links ]
2. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol 2002, 186:1160—1166. [ Links ]
3. Lawrence JM, Lukacz ES, Nager CW, Hsu JW, Luber KM. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol 2008, 111:678-685. [ Links ]
4. Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Prevalence of symptomatic pelvic organ prolapse in a Swedish population. Int Urogynecol J Pelvic Floor Dysfunct 2005, 16:497—503. [ Links ]
5. DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller A. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol 2007, 109:295-302. [ Links ]
6. Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG 2008, 115:979-984. [ Links ]
7. Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am 1998, 25:723-746. [ Links ]
8. Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet 2007, 369:1027-1038. [ Links ]
9. Chen L, Ashton-Miller JA, Hsu Y, DeLancey JO. Interaction among apical support, levator ani impairment and anterior vaginal wall prolapse. Obstet Gynecol 2006, 108:324-332. [ Links ]
10. Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol 2006, 195(6):1837-1840. [ Links ]
11. Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol 2003, 189(2):372-377. [ Links ]
12. Barber MD, Brubaker L, Nygaard I, Wheeler TL 2nd, Schaffer J, Chen Z, Spino C, Pelvic Floor Disorders Network. Defining success after surgery for pelvic organ prolapse. Obstet Gynecol 2009, 114(3):600-609. [ Links ]
13. Barber MD, Walters MD, Cundiff GW, PESSRI Trial Group. Responsiveness of the Pelvic Floor Distress Inventory (PFDI) and Pelvic Floor Impact Questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. Am J Obstet Gynecol 2006, 194(5):1492-1498. [ Links ]
14. Flynn BJ, Webster GD. Surgical management of the apical vaginal defect. Curr Opin Urol 2002, 12(4):353-358. [ Links ]
15. Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol 1999, 180:859—865. [ Links ]
16. Sze EH, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol 1997, 89:466-475. [ Links ]
17. Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol 2013, 121(2 Pt 1):354-374. [ Links ]
18. Paraiso MF, Ballard LA, Walters MD, Lee JC, Mitchinson AR. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Obstet Gynecol 1996, 175:1423-1430. [ Links ]
19. Morgan DM, Rogers MA, Huebner M, Wei JT, DeLancey JO. Heterogeneity in anatomic outcome of sacrospinous ligament fixation for prolapse: a systematic review. Obstet Gynecol 2007, 109(6):1424-1433. [ Links ]
20. Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol 2010, 202(2):124-134. [ Links ]
21. Edenfield AL, Amundsen CL, Weidner AC, Wu JM, George A, Siddiqui NY. Vaginal prolapse recurrence after uterosacral ligament suspension in normal-weight compared with overweight and obese women. Obstet Gynecol. 2013, 121(3):554-559. [ Links ]
22. Flynn MK, Weidner AC, Amundsen CL. Sensory nerve injury after uterosacral ligament suspension. Am J Obstet Gynecol 2006, 195(6):1869-1872. [ Links ]
23. Montoya TI, Luebbehusen HI, Schaffer JI, Wai CY, Rahn DD, Corton MM. Sensory neuropathy following suspension of the vaginal apex to the proximal uterosacral ligaments. Int Urogynecol J 2012, 23(12):1735-1740. [ Links ]
24. Abbasy S, Kenton K. Obliterative procedures for pelvic organ prolapse. Clin Obstet Gynecol 2010, 53:86-98. [ Links ]
25. Barber MD, Amundsen CL, Paraiso MF, Weidner AC, Romero A, Walters MD. Quality of life surgery for genital prolapse in elderly women: Obliterative and reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct 2007, 18:799-806. [ Links ]
26. DeLancey JO, Morley GW. Total colpocleisis for vaginal eversion. Am J Obstet Gynecol 1997, 176:1228-1232. [ Links ]
27. Zebede S, Smith AL, Plowright LN, Hedge A, Aguilar VC, Davila GW. Obliterative LeFort colpocleisis in a large group of elderly women. Obstet Gynecol 2013, 121(2 Pt 1):279-284. [ Links ]
28. FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H, Ann Weber for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct 2006, 17(3):261-266. [ Links ]
29. Hoffman MS, Cardosi RJ, Lockhart J, Hall DC, Murphy SJ. Vaginectomy with pelvic herniorrhaphy for prolapse. Am J Obstet Gynecol 2003, 189(2):364-367. [ Links ]
30. von Pechmann WS, Mutone M, Fyffe J, Hale DS. Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol 2003, 189(1):121-125. [ Links ]
31. Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2013, Issue 4 Art. No: CD004014. DOI: 10.1002/14651858.CD004014.pub5.
32. Vu MK, Letko J, Jirschele K, Gafni-Kane A, Nguyen A, Du H, Goldberg RP. Minimal mesh repair for apical and anterior prolapse: initial anatomical and subjective outcomes. Int Urogynecol J 2012, 23(12):1753-1761. [ Links ]
33. Davila GW, Baessler K, Cosson M, Cardozo L. Selection of patients in whom vaginal graft use may be appropriate. Consensus of the 2nd IUGA Grafts Roundtable: Optimizing Safety and Appropriateness of Graft Use in Transvaginal Pelvic Reconstructive Surgery. Int Urogynecol J 2012, 23(Suppl 1):S7-14. [ Links ]
34. Cox A, Herschorn S. Evaluation of Current Biologic Meshes in Pelvic Organ Prolapse Repair. Curr Urol Rep 2012, 13:247—255. [ Links ]
35. Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol 2005, 106(1):29-37. [ Links ]
36. Nygaard IE, McCreery R, Brubaker L, Connolly A, Cundiff G, Weber AM, Zyczynski H, Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol 2004, 104(4):805-823. [ Links ]
37. Cundiff GW, Varner E, Visco AG, Zycynski HM, Nager CW, Norton PA, Schaffer J, Brown MB, Brubaker L, Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol 2008, 199(6):688. e1-5. [ Links ]
38. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet Gynecol 2009, 133(2 Pt 1): 367-373. [ Links ]
39. Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol 2005, 192(5)1752-1758.
40. Higgs PJ, Chua HL, Smith AR. Long term review of laparoscopic sacrocolpopexy. BJOG 2005, 112(8):1134-1138. [ Links ]
41. Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic and abdominal sacrocolpopexy. J Minim Invasive Gynecol 2010, 17(4):493-499. [ Links ]
42. Paraiso MF, Jelovsek JE, Frick A, Chen CC, Barber MD. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol 2011, 118(5):1005-1013. [ Links ]
43. Anger JT, Mueller ER, Tarnay C, Smith B, Stroupe K, Rosenman A, Brubaker L, Bressee C, Kenton K. Robotic compared with laparoscopic sacrocolpopexy: A randomized controlled trial. Obstet Gynecol 2013, 123:5-12. [ Links ]
44. Ridgeway B, Frick AC, Walters MD. Hysteropexy: A review. Minerva Ginecol 2008, 60:509-528. [ Links ]
45. Krause HG, Groh JT, Sloane K, Higgs P, Carey MP. Laparoscopic sacral suture hysteropexy for uterine prolapse. Int Urogynecol J Pelvic Floor Dysfunc 2006, 17:378-381. [ Links ]
46. van Brummen HJ, van de Pol G, Aalders CI, Heintz AP, van der Vaart CH. Sacrospinous hysteropexy compared to vaginal hysterectomy as primary surgical treatment fro a descensus uteri: effects on urinary symptoms. Int J Urogynecol 2003, 14:350-355.
47. Constantini E, Mearini L, Bini V, Zucchi A, Mearini E, Porena M. Uterus preservation in surgical correction of uterovaginal prolapse. Eur Urol 2005, 48:642-649. [ Links ]
48. Hefni M, El-Toukhy T, Bhaumik J, Katsimanis E. Sacrospinous cervicocolpopexy with uterine conservation for uterovaginal prolapse in elderly women: an evolving concept. Am J Obstet Gynecol 2003, 188:645-650. [ Links ]
49. Collinet P, Belot F, Debodinance P, Ha Duc E, lucot JP, Cosson M. Transvaginal mesh technique for pelvic organ prolapse repair: mesh exposure management and risk factors. Int Urogynecol J Pelvic Floor Dysfunct 2006, 17:315-320. [ Links ]
50. Wu J, Wells E, Hundley A, Connolly A, Williams KS, Visco AG. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol 2006, 194:1418-1422. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Inês Pereira
E-mail: ines.ifpereira@gmail.com
Recebido em: 29/7/2015
Aceite para publicação: 19/12/2015