Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Obstétrica e Ginecológica Portuguesa
versão impressa ISSN 1646-5830
Acta Obstet Ginecol Port vol.11 no.3 Coimbra set. 2017
REVIEW ARTICLE/ARTIGO DE REVISÃO
Fetal repercussions of Zika virus infection during pregnancy
Repercussões fetais da infeção por Zika durante a gravidez
Catarina Lopes de Almeida*, Carla Ramalho**
Faculdade de Medicina da Universidade do Porto
*Mestrado Integrado em Medicina, Faculdade de Medicina da Universidade do Porto
**MD, PhD, Departamento de Ginecologia-Obstetrícia e Pediatria, Faculdade de Medicina da Universidade do Porto, Serviço de Ginecologia e Obstetrícia, Centro Hospitalar S. João, Instituto de Investigação e Inovação em Saúde, Porto
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Recently, Zika virus infection has been rapidly spreading, rising the cases of fetal abnormalities. A review was performed from February 2016 to January 2017, selecting articles that evaluated the fetal repercussions of maternal infection, considering fetal autopsies, clinical examinations and imagiology. The most frequent abnormalities were in the central nervous system, namely microcephaly, intracranial calcifications and ventriculomegaly. Death and fetal growth restriction were also reported. Anomalies in other systems were found to a lesser extent. Despite enough evidence proving this causality, it is important to document all these changes, allowing the comprehension of the virus' full width.
Keywords: Zika virus; Zika virus infection; Pregnancy; Microcephaly; Emerging infectious diseases.
Introduction
ZIKV belongs to the Flaviviridae family, which includes several other clinically significant viruses, such as dengue, West Nile and yellow fever viruses1. It is transmitted to humans through the bite of the infected mosquitoes, being the Aedes genus the most frequent vector2.
Structurally, ZIKV is a positive-sense single-stranded RNA virus which contains 10,794 nucleotides encoding 3,419 amino acids3. This virus consists of two non-coding regions that flank an open reading frame which encodes the capsid protein, envelope glycoprotein, the membrane or precursor membrane protein, and other seven non-structural proteins involved in replication, assembly and suppression of the host's natural immunological response4.
Phylogenetic studies reveal the existence of two main lineages, African and Asian, being that the latter originated in the course of the virus's migration from East Africa to Southeast Asia; from here, the virus spread to the Pacific Islands and French Polynesia, among other countries, all the way to the Americas5 (Silva A.)
The virus was first isolated in 1947 from a rhesus monkey in the Zika forest in Uganda during the research into the epidemiology of yellow fever and was isolated from Aedes africanus mosquitoes the following year6. Later on, in 1952, the virus was isolated from humans also in Uganda and in Nigeria7. In the same decade, the species Aedes aegypti was identified as the most common vector for ZIKV transmission8.
Ever since its discovery, some cases of ZIKV infection in humans in Africa, India and Southeast Asia have been reported, although they were isolated and separated in time. The first noteworthy outbreak was reported in 2007 on Yap Island in the Federated States of Micronesia9. In this outbreak, it was estimated that around 73% of the island's population was infected with ZIKV and some of the symptoms included rash, fever, arthralgia and conjunctivitis; however, there were no hospitalizations or deaths9. Another striking outbreak took place in French Polynesia between October 2013 and April 2014, this time infecting 66% of the population10. Due to the contemporaneous increase of Guillain-Barré syndrome during that outbreak, retrospective studies were made and successfully proved the association between ZIKV infection and Guillain-Barré syndrome11. These were the first documented neurologic sequelae caused by ZIKV infection. Subsequently, in 2014, the ZIKV disseminated through some of the South Pacific islands, including Easter Island, the Cook Islands and New Caledonia12. The most recent and significant outbreak was in northeastern Brazil in late 2014, which rapidly and surprisingly spread throughout the whole country; alongside with it, cases of Guillain-Barré syndrome and microcephaly significantly increased as well.13 Since then, ZIKV quickly disseminated throughout Central and South America.
Considering this fast dissemination and its apparent consequences, on February 1st of 2016, the WHO declared ZIKV infection a Public Health Emergency of International Concern14. As of January 2017, this problem continues to evolve, bringing uncertainty about the repercussions of this global threat.
Although the main cause for ZIKV infection is a bite from an infected mosquito, some cases of sexual and perinatal transmission have been reported10. In adults, this infection is mostly asymptomatic (80%)15. When symptoms occur, they are mild and non-specific, and include: pruritic maculopapular rash, mild fever, arthralgia, myalgia, headache, non-purulent conjunctivitis and retro-orbital pain. Usually there is a full recovery within 1-2 weeks of onset10, 15. Deaths are not common in this infection and are mostly limited to immunocompromised patients or in those who already have other comorbidities16,17.
The prevalence of Zika virus (ZIKV) has been recently increasing. There have been outbreaks since 2007, the most recent and significant one being in Brazil at the end of 2014. Since then, ZIKV has spread to Central and South America, and as of January 2017 there were 51 countries with active transmission18. This expansion of the virus' transmission has led to a large increase of cases not only of microcephaly, but also other abnormalities within and outside the central nervous system (CNS). The objective of this review of the literature is to describe all documented fetal structural abnormalities associated with ZIKV published to date.
Methods
A review of the literature was carried out from February 2016 (when the WHO declared the ZIKV infection a Public Health Emergency) until January 2017, using the MEDLINE database. For the initial research on the virus, we had no restriction on the publication year, and looked for articles with the keywords “Zika virus structure”, “Zika virus pathogenesis” and “Zika virus outbreaks”. The focus of the investigation were the fetal repercussions of the ZIKV infection; therefore, we looked for articles whose evaluations took into account fetal autopsies, clinical examinations and imaging techniques during pregnancy and/or in the live births. In the beginning of the research, there was very little information on this topic, since it was arising at the moment. However, during its course, more and more information was available. Consequently, we restricted it to some keywords such as “Zika virus and pregnancy”, “Zika virus and microcephaly” or “fetal repercussions of Zika virus”. We excluded every article that did not focus on the fetal repercussions of the ZIKV infection, such as the ones about consequences of this infection in the pregnant woman, or those based merely on suspicions, without clinical examinations, imaging techniques or autopsies. The case review articles were all published from 2016 onwards.
Case reviews of fetal infection
Between October 2015 and May 2016, 7,534 suspected cases of microcephaly in Brazil have been reported, 1,384 of which were confirmed; of these, 207 had in fact ZIKV infection, which was laboratory-confirmed19. Of all the 7,534 suspected cases of microcephaly, there were 273 intrauterine or neonatal deaths, 59 of which were actually confirmed to have microcephaly or other CNS malformation19.
Colombia is the second most affected country by the ZIKV infection in the American continent, and as of October 2016, there were 95,898 suspected and 8,826 confirmed cases of ZIKV infection; out of the reported cases of pregnant women with confirmed and suspected ZIKV infection, 47 cases of microcephaly were associated with ZIKV infection, 213 cases were excluded and 342 cases are under investigation20. There was an increase in the incidence of congenital malformations in September and a tendency to increase even more in the subsequent months.
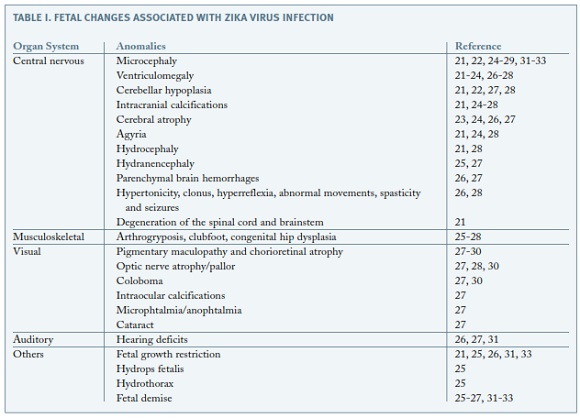
During the period of the research, 13 articles reporting fetal anomalies due to ZIKV were found. The anomalies found in several organ systems are summarized in Table I.
The first ever confirmed case of this association was reported in February 2016; a 25 year old Slovenian woman was infected with ZIKV in Brazil at the end of the first trimester of pregnancy21. The patient had symptomatic disease during the 13th week of gestation and the pregnancy carried on without complications until the 29th week, when she noticed reduced fetal movements. The ultrasonography at 32 weeks showed fetal growth restriction, microcephaly, ventriculomegaly, a diminished trancerebellar diameter and several calcifications spread throughout the brain and the placenta21. The pregnancy was terminated and the autopsy revealed microcephaly, open sylvian fissures, agyria, internal hydrocephaly of the lateral ventricles and calcifications in the cortex and subcortical white matter in the frontal, occipital and parietal lobes21. Nonetheless, the cytoarchitecture of the fetal brain was preserved. There was also diffuse astrogliosis with focal astrocytic outburst into the subarachnoid space. The brain stem and spinal cord suffered from degeneration in the long descending tracts, predominantly in the lateral corticospinal tract. 6.5x107 ZIKV RNA copies per milligram of tissue were identified exclusively in the brain, with no further lesions in any other organ, which demonstrates this virus' neurotropism21. Some of these alterations in the brain were also reported in the same month by ultrasonography at 21 and 22 weeks of gestation in women who had ZIKV infection symptoms22. About one month later, a case of a confirmed infection of a Finnish woman in Central America during the 11th week of pregnancy was described; at 20 weeks of gestation, the fetal head circumference had decreased from the 47th to the 24th percentile and ultrasonography and magnetic resonance imaging showed cerebral atrophy and ventriculomegaly23. One month after this last case, another one was described in Brazil, and it exposed some of the same brain anomalies, such as microcephaly, ventriculomegaly, intracranial calcifications, cerebral atrophy and agyria24.
In Salvador, Brazil, a case of a 20 year old pregnant woman was reported. The ultrasound at 26 weeks detected fetal growth restriction, microcephaly, hydranencephaly, brain calcifications, hydrothorax, ascites and subcutaneous edema25. Once again, the virus was only identified in the CNS (cerebral cortex, medulla oblongata and cerebrospinal fluid) and placenta. An induced labour was performed at 32 weeks due to fetal demise and the stillbirth showed signs of arthrogryposis25.
A case-control study from Rio de Janeiro, Brazil, enrolled 345 women who described acute febrile illness with a rash during pregnancy and were followed to determine pregnancy and infants outcomes, comparing cases positive for ZIKV infection during pregnancy (53%) to those who were negative26. Some of the described abnormalities included cerebral calcifications, cerebral atrophy, ventricular augmentation and hypoplasia of cerebral structures. These adverse outcomes happened in fetuses of women who were infected between 6 and 39 weeks of gestation, except the calcifications, which were only seen in infants whose mothers were infected at a maximum of 34 weeks26. Cerebral parenchyma hemorrhages were also detected, and in one case the mother had been infected at 39 weeks. Out of the live births, 42% had abnormalities on imaging or examination, and out of those, 63% had grossly abnormal neurological examinations, showing hypertonicity, clonus, hyperreflexia, spasticity and seizures26. 9% of the fetuses exposed to ZIKV and 5.3% of fetuses in the control group were small for gestational age (p=0.06). Microcephaly was detected in 2 infants exposed to ZIKV (1.7%), both infected in the first trimester of pregnancy, and it was not displayed in any of the infants of the control group26. This study confirms that ZIKV infection causes severe and recurrent problems in the CNS development in utero, as well as in general fetal development, being that in this cohort, such problems affected 46% of fetuses and 42% of live-born infants26.
Although the most frequent and best studied alterations were those in the CNS, an increasing number of studies has been arising, suggesting the impact of the ZIKV in other systems. The involvement of the musculoskeletal system was documented in several cases. In the Salvador case, the fetal autopsy showed signs of arthrogryposis25. In the case-control study in Rio, foveas in the elbows and knees as a result of limb contractures in utero were reported26. Another study also described the presence of musculoskeletal anomalies secondary to CNS dysfunction, such as arthrogryposis, clubfoot and congenital hip dysplasia27. The latter was reported in a case in Pernambuco as well28.
Reports of ocular abnormalities have also been progressively increasing. Chorioretinal anomalies (involving or not the macula) were the most prevalent ones, suggesting the children could have reduced vision. The ophthalmological exams revealed alterations in the macula, such as chorioretinal atrophy, pigment stippling and focal pigment mottling of the retina26-30. Optic nerve atrophy was detected in three studies through pallor in the ocular assessment27,28,30. Other defects consisted in coloboma, intraocular calcifications, microphtalmia, anophtalmia and cataracts27,30. One of these studies detected ocular abnormalities in 10 of 29 patients with microcephaly, whose mothers had had ZIKV infection during pregnancy (34.5%)30. However, a causal link has not been established for all the ocular anomalies. Hearing deficits were also reported26,27,31.
Fetal demise appears to be a consistent bad outcome25-27,31-33. The Salvador case described above suggested there could be a link between ZIKV infection and hydrops fetalis and fetal demise, although the mechanism remains unknown25.
Likewise, fetal growth restriction has also been documented as a prevalent result in the event of maternal ZIKV infection during pregnancy.21,26,31,33.
Conclusion
There has been sufficient evidence to establish a causal relationship between the ZIKV infection during pregnancy and neonatal structural anomalies21,34. Besides this fact, there has not been any other explanation for the defects detected in the studies mentioned above and there is also documented proof in tissue biopsies of congenital ZIKV infection; along with that, other viruses that could have the same repercussions have been excluded in the majority of studies. Despite extensive research, there is no alternative hypothesis for the microcephaly and all the other brain defects observed.
The timeframe in order for malformations to occur is throughout the pregnancy; although only the fetuses whose mothers were infected in the first trimester had malformations related to embryogenesis, abnormalities in the CNS were detected in fetuses infected until 39 weeks of gestation26.
Even though the most frequent anomalies were those involving the CNS, other systems have also been affected. Microcephaly has been the most discussed fetal malformation when it comes to ZIKV infection. This may be, in part, because this virus is more active in developing countries, meaning more precarious diagnostic methods and a delayed search for medical assistance. Therefore, it could be the most common abnormality simply because it is easily perceived in the clinical exam, while other defects may go unnoticed. There have been other prevalent findings in the CNS, such as ventriculomegaly, cerebral and cerebellar hypoplasia and intracranial calcifications, among others. In nearly all the studies, fetal growth restriction and fetal demise were documented. Although in a smaller proportion, anomalies found in other systems have proven to be more prevalent and should also be a major source of concern. These consist of hearing deficits, hydrothorax and hydrops fetalis. Nevertheless, the most frequent alterations outside the CNS were optical.
After establishing these causal relationships, other concerns take place. First of all, it is urgent to determine the full extension of the malformations; if there are similarities between ZIKV and other teratogens, there could be a phenotype expansion and more defects could exist besides the ones we know of at the moment. In second place, it is important to clear the differences in the birth defects according to the different periods of time in pregnancy during which the mother was infected. Finally, determining factors that may alter the outcome of an already adverse pregnancy is also relevant, such as severity of the infection, coinfection with another virus, previous contact with another flavivirus causing an existing immune response, and also the genetic background of the mother and the fetus34.
REFERENCES
1. Plourde AR BE. A literature review of Zika virus. Emerg Infect Dis 2016 [ Links ]
2. Doença do Vírus Zika, Orientação nº 001/2016 de 15/01/2016 atualizada a 02/06/2016.
3. Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152(4):687-696. [ Links ]
4. Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 A resolution cryo-EM structure of Zika virus. Science. 2016;352(6284):467-470. [ Links ]
5. Gatherer D, Kohl A. Zika virus: a previously slow pandemic spreads rapidly through the Americas. Journal of General Virology. 2016;97(2):269-273. [ Links ]
6. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509-520. [ Links ]
7. Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139-145. [ Links ]
8. Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50(5):442-448. [ Links ]
9. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009; 360(24):2536-2543. [ Links ]
10. Lessler J, Chaisson LH, Kucirka LM, Bi Q, Grantz K, Salje H, Carcelen AC, Ott CT, Sheffield JS, Ferguson NM, Cummings DAT, Metcalf CJE, Rodriguez-Barraquer I. Assessing the global threat from Zika virus. Science. 2016. [ Links ]
11. Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial A-L, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra J-C, Despres P, Fournier E, Mallet H-P, Musso D, Fontanet A, Neil J, Ghawché F. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. The Lancet.387(10027):1531-1539.
12. Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, Guillaumot L, Souares Y. Concurrent outbreaks of dengue, chikungunya and Zika virus infections - an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill. 2014;19(41).
13. Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT. Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59-62. [ Links ]
14. WHO. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/2016 [cited 2016 Sep 7]. [ Links ]
15. Mlacker S, Shafa G, Aldahan AS, Shah VV, Samarkandy S, Nouri K. Origin of the Zika virus revealed: a historical journey across the world. Int J Dermatol. 2016. [ Links ]
16. L. Arzuza-Ortega AP, G. Pérez-Tatis, H. López-García, E. . Fatal sickle cell disease and Zika virus infection in girl from Colombia. . Emerg Infect Dis. (22):925-927. [ Links ]
17. Sarmiento-Ospina A, Vasquez-Serna H, Jimenez-Canizales CE, Villamil-Gomez WE, Rodriguez-Morales AJ. Zika virus associated deaths in Colombia. Lancet Infect Dis. 2016;16(5):523-524. [ Links ]
18. CDC. Countries and Territories with Active Local Zika Virus Transmission http://www.cdc.gov/zika/geo/active-countries.html 2016 [cited 2016 December]. [ Links ]
19. Control. ECfDPa. Rapid Risk Assessment. Zika virus disease epidemic: Sixth update. Stockholm: ECDC; 2016 [updated 20 May 2016; cited 2016 5 October]. [ Links ]
20. Control ECfDPa. Rapid Risk Assessment. Zika virus disease epidemic: Ninth update Stockholm: ECDC; 2016 [updated 28 October 2016; cited 2016 December]. [ Links ]
21. Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodušek V, Vizjak A, Pižem J, Petrovec M, Avšič Županc T. Zika Virus Associated with Microcephaly. New England Journal of Medicine. 2016;374(10):951-958.
22. Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, Araujo ESM, de Sequeira PC, de Mendonça MCL, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, dos Santos FB, Nogueira RMR, Tanuri A, de Filippis AMB. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. The Lancet Infectious Diseases.16(6):653-660.
23. Driggers RW, Ho C-Y, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. New England Journal of Medicine. 2016;374(22):2142-2151. [ Links ]
24. Werner H, Fazecas T, Guedes B, Lopes Dos Santos J, Daltro P, Tonni G, Campbell S, Araujo Júnior E. Intrauterine Zika virus infection and microcephaly: correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound in Obstetrics & Gynecology. 2016;47(5):657-660. [ Links ]
25. Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, Santos LA, Nery N, Jr., Vasilakis N, Ko AI, de Almeida AR. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis. 2016;10(2):e0004517.
26. Brasil P, Pereira JPJ, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai U-A, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baião AE, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. New England Journal of Medicine. 2016;375(24):2321-2334. [ Links ]
27. Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, Ellington SR, Shapiro-Mendoza CK, Oduyebo T, Fine AD, Brown CM, Sommer JN, Gupta J, Cavicchia P, Slavinski S, White JL, Owen SM, Petersen LR, Boyle C, Meaney-Delman D, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. Jama. 2017;317(1):59-68. [ Links ]
28. Culjat M, Darling SE, Nerurkar VR, Ching N, Kumar M, Min SK, Wong R, Grant L, Melish ME. Clinical and Imaging Findings in an Infant With Zika Embryopathy. Clinical Infectious Diseases. 2016;63(6):805-811. [ Links ]
29. Miranda HAd, II, Costa MC, Frazão MAM, Simão N, Franchischini S, Moshfeghi DM. Expanded Spectrum of Congenital Ocular Findings in Microcephaly with Presumed Zika Infection. Ophthalmology.123(8):1788-1794.
30. de Paula Freitas B, de Oliveira Dias J, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed zika virus congenital infection in salvador, brazil. JAMA Ophthalmology. 2016;134(5):529-535. [ Links ]
31. Pacheco O, Beltrán M, Nelson CA, Valencia D, Tolosa N, Farr SL, Padilla AV, Tong VT, Cuevas EL, Espinosa-Bode A, Pardo L, Rico A, Reefhuis J, González M, Mercado M, Chaparro P, Martínez Duran M, Rao CY, Muñoz MM, Powers AM, Cuéllar C, Helfand R, Huguett C, Jamieson DJ, et al. Zika Virus Disease in Colombia - Preliminary Report. New England Journal of Medicine.0(0):null.
32. Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, Araujo de Franca GV. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(9):242-247. [ Links ]
33. Pomar L, Malinger G, Benoist G, Carles G, Ville Y, Rousset D, Hcini N, Pomar C, Jolivet A, Lambert V. Association between Zika virus and foetopathy: a prospective cohort study in French Guiana. Preliminary report. Ultrasound Obstet Gynecol. 2017. [ Links ]
34. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects - Reviewing the Evidence for Causality. New England Journal of Medicine. 2016;374(20):1981-1987. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Catarina Lopes de Almeida
Albergaria-a-Velha, Portugal
E-mail: catarina1313@gmail.com
Recebido em: 26/03/2017
Aceite para publicação: 15/06/2017