Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Obstétrica e Ginecológica Portuguesa
versão impressa ISSN 1646-5830
Acta Obstet Ginecol Port vol.13 no.2 Coimbra jun. 2019
ORIGINAL STUDY/ESTUDO ORIGINAL
sFlt-1/PlGF ratio for the predictive diagnosis of preeclampsia: budget impact analysis from the public healthcare perspective in Portugal
sFlt-1/PIGF no diagnóstico preditivo de pré-eclâmpsia: estudo de impacto económico em Portugal
Ana Campos1, Ana Paula Machado2, Helena Martins3, Maria São José Pais4, Katalin Érsek5, Nelson Lopes6
1 MD, PhD, Departamento de Medicina Materno-Fetal; Maternidade Alfredo da Costa
2 MD, Serviço de Ginecologia e Obstetrícia; Centro Hospitalar Universitário S. João - Porto
3 MD, Serviço de Química Clínica - Departamento de Patologia; Centro Hospitalar do Porto
4 MD, Serviço de Obstetrícia A; Centro Hospitalar Universitário de Coimbra
5 MsC, Healthcare Development, Roche Diagnostics
6 MD, Departamento Médico, Roche Diagnostics
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Overview and Aims: The last decade brought relevant insights into the pathophysiology of preeclampsia (PE), namely the role of the circulating levels of placental growth factor (PlGF) and soluble Fms-like tyrosine kinase-1 (sFlt-1). The purpose of this study is to estimate the financial impact of introducing the sFlt-1/PlGF ratio for the evaluation of women with suspicion of PE in the Portuguese National Healthcare System (SNS).
Study Design: budget impact study evaluating short-term costs associated with the introduction of the sFlt-1/PlGF ratio from the SNS payer’s perspective. The time horizon for the study is 1 year.
Population: The target population consists of women presenting to the healthcare system with signs or symptoms suggestive of preeclampsia (estimated in 8500 subjects).
Methods: A decision-tree model was used to estimate the budget impact of the introduction of the sFlt-1/PlGF ratio in the SNS. The model compares the management costs in the current clinical practice ("no test" scenario) vs. current diagnostic procedures plus the sFlt-1/PlGF ratio ("test" scenario). Clinical inputs have been derived primarily from literature review and, where data was unavailable, expert opinion. Resources and unit costs have been obtained from Portugal-specific sources.
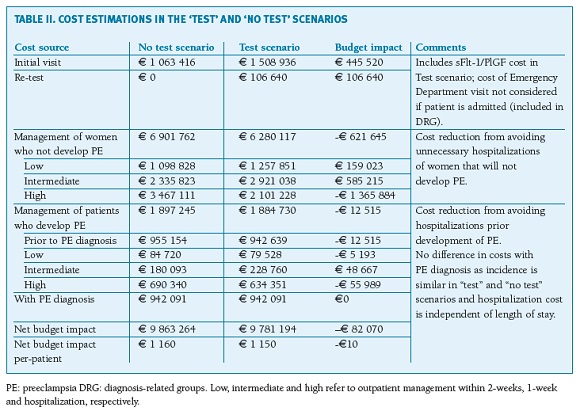
Results: In the current standard practice (no test), total costs were estimated to be e9 863 264 (e1160 per patient), with unnecessary admissions representing about e3,5 million. Total costs in the test scenario sum up to e9 781 194 (e1150 per patient), representing a cost saving to the system of e82 070 (e10 per patient), mainly due to a reduction of false positives and related unnecessary hospitalizations of women not developing PE.
Conclusions: There is favorable economic evidence about the introduction of the sFlt- 1/PlGF ratio in the SNS. The generated savings appear to offset the costs related to the test.
Keywords: Preeclampsia; Pregnancy; Biomarkers; Costs and Cost Analysis.
Introduction
Hypertension is the most common medical problem found in pregnancy. It affects 5-15% of all pregnancies, with preeclampsia (PE) and related disorders comprising the most feared complications. Severe complications of PE can be catastrophic, representing one of the leading causes of maternal and neonatal morbidity and mortality1-5. In Portugal, a large national survey from 2005, estimated the prevalence of hypertensive disorders during pregnancy in 6%. PE, eclampsia and the Hemolysis, Elevated Liver enzymes and Low Platelet count (HELLP) syndrome summed up to 1,7%6. More recent cohorts show a prevalence of PE between 2,6-2,9%, a rate closer to that reported by other developed countries 7-10.
The primary method for PE diagnosis relies on the presentation of hypertension and proteinuria. However, clinical presentation, and particularly the dynamics of the clinical course, can vary significantly, making the definite diagnosis uncertain11,12. Further, these clinical variables perform poorly in predicting PE related adverse outcomes13. Uncertainty in confirming diagnosis may lead to unnecessary admissions, inappropriate hospital discharges, iatrogenic preterm delivery and, ultimately, excess healthcare expenditure14. Consequently, there remains a need for diagnostic tests that can accurately identify patients who develop PE and those at risk for adverse outcomes. Patients identified as low risk for developing PE could be safely followed in an outpatient setting, while those at moderate or high risk would be managed more cautiously, eventually admitted or referred to a tertiary center15,16.
The last decade brought relevant insights into the pathophysiology of PE, particularly regarding the role of the angiogenic balance in the disease. Circulating soluble Fms-like tyrosine kinase-1 (sFlt-1) levels are markedly increased, whereas placental growth factor (PlGF) levels are decreased, resulting in a net anti-angiogenic state that directly contributes to the cascade of the preeclamptic syndrome17-20. The imbalance in the concentrations of sFlt-1 and PlGF is found several weeks prior to the onset of clinical manifestations. A large number of studies show that raised sFlt-1/PlGF ratios are strongly associated with underlying PE while low values rule out the development of the disease21-28.
The automated assays to measure sFlt-1 and PlGF (Elecsys® sFlt-1/PlGF ratio; Roche Diagnostics) became recently available in Portugal. Besides the clinical utility, decision-makers need to assess the benefits and costs of new technologies to appreciate the increased value for patients and perform estimates of the financial sustainability for the healthcare system. The purpose of this study was to estimate the financial impact of introducing the sFlt-1/PlGF ratio for the evaluation of women with suspicion of PE in the Portuguese National Healthcare System (Serviço Nacional de Saúde; SNS).
Methods
Study Design
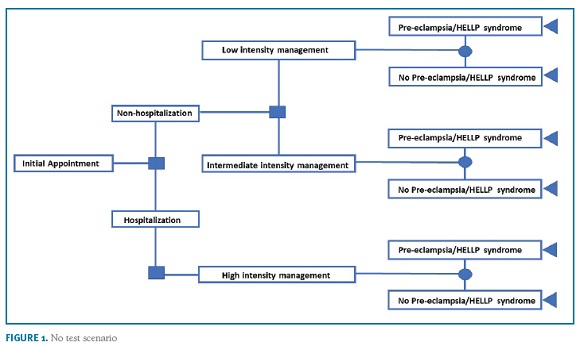
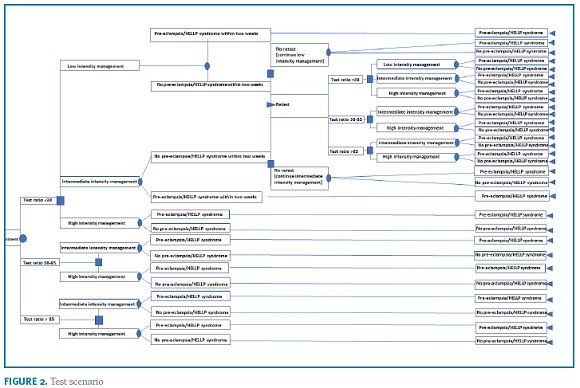
A decision-tree model was used to estimate the budget impact of the introduction of the sFlt-1/PlGF ratio for the evaluation of women with clinical suspicion of PE from the SNS payer’s perspective. The model compares the management costs in the current clinical practice ("no test" scenario) vs. current diagnostic procedures plus the sFlt-1/PlGF ratio ("test" scenario). The research hypothesis is that the information provided by the test may decrease the hospitalization rate, particularly unnecessary hospitalizations of women that will not develop PE, thereby reducing costs for the system. The model simulates the clinical pathway of a patient presenting with signs or symptoms of PE, and who is then managed in either an inpatient or an outpatient setting. In the two model arms, a three-level decision pathway was modelled to reflect clinical practice. Women with high suspicion for PE are admitted into the hospital (eg moderate/severe hypertension, severe proteinuria, hepatic dysfunction, low platelets). Patients not admitted at first visit are managed in two outpatient care intensity levels: women estimated to be at a low risk (eg mild isolated symptoms in women with no prior risk, mild gestational hypertension) are re-evaluated at specialist appointment within 2-weeks; women estimated to be at intermediate risk (eg worsening of chronic hypertension or proteinuria; abnormal utero-placental perfusion; autoimmune diseases) are re-evaluated within 1-week. The algorithm for the "test scenario" is based on expert consensus on the use of the sFlt-1/PlGF ratio29,30,31. Women with raised ratio (>85 [110 for late-onset PE]) are at very high-risk for developing the disease and will require hospitalization; women with negative results (<38) are at low risk for developing PE and the vast majority can be managed in outpatient setting with specialist appointment within 2-weeks; women with intermediate results (38-85 [110]) are at moderate risk to develop PE and, depending on the individual clinical presentation, need close monitoring if managed in outpatient setting (specialist appointment up to 1 week) or hospitalization. An option for retesting is considered for women with initial negative results that have not developed PE but may still present clinical signs or symptoms of disease (2 weeks after initial visit). The algorithms are shown in Figure 1 and 2. The time horizon for the study is one year.
Population
The target population consists of women presenting to the healthcare system with signs or symptoms suggestive of PE (eg new onset of elevated blood pressure; aggravation of pre-existing hypertension; new onset of proteinuria; epigastric pain; severe edema; headache; visual disturbances; weight gain; low platelets; elevated liver enzymes; suspected intrauterine growth restriction; abnormal utero-placental perfusion). The target population was estimated in 8500 subjects, considering the number of deliveries reported in Portugal during year 2016 (85444)32 and assuming a prevalence of hypertensive disorders during pregnancy between 5% to 15% with a mean of 10%1-5.
Model inputs
PROGNOSIS26 was a prospective, non-interventional study conducted across 30 sites globally, aimed to derive and validate a cut-off of the sFlt-1/PlGF ratio for the short-term prediction of PE. A total of 1050 women with suspected PE between 24 + 0 and 36 + 6 weeks’ gestation were enrolled. In this study, data from PROGNOSIS was used to populate the model parameter estimations, including rates of hospitalized women in the absence of test information (‘no-test’ scenario), the correlation between the test ratio and hospitalization (‘test’ scenario), and the relationship between test ratio, hospitalization and development (or not) of PE.
For the ‘no test’ scenario a hospitalization rate of 36.1% was assumed. The figure is inputted from PROGNOSIS, as the ratio levels were blinded to investigators and patients, therefore not affecting standard clinical practice. Non-hospitalized women were equally split between low and intermediate management levels.
In the ‘test’ scenario women are classified into three subgroups based on the sFlt-1/PlGF results (<38; 38-85 [110]; >85 [110]). Analysis of the test information from PROGNOSIS showed that 76.1%, 10.7% and 13.2% of subjects fall on each sub groups, respectively. It is assumed in the model that, for a test ratio <38, some women (eg blood pressure higher than 160/110 mmHg) would still be hospitalized. Data from PROGNOSIS shows that 1.7% of women met these criteria. As the blood pressure threshold for hospitalization in Portugal is usually lower, the hospitalization rate for women with results <38 was assumed as 5% (expert opinion). For non-hospitalized women, a conservative equal split between low and intermediate management levels was considered. For the subgroups of women with ratios >38, as the knowledge of a positive result may lead to a safer approach, the base case analysis considers a reflex increase of hospitalizations relative to PROGNOSIS data: 55% to 75% and 65% to 85% for women with results 38-85 [110] or >85 [110], respectively (expert opinion). The resources allocated to the low and intermediate outpatient management levels are based on conservative local practice patterns. Re-testing was considered in 40% of the women with an initial negative result that did not develop PE within 2 weeks of the first visit (expert opinion). The default values for estimates of unplanned emergency visits and admission to neonatal intensive care are derived from the PROGNOSIS data. In the absence of evidence, the model assumes that information from the test has no effect on these variables.
Costs
The model estimates costs to the healthcare system (inpatient and outpatient care) associated with diagnosis, monitoring and management from first presentation of suspected PE (week 32, on average) until the development of PE, HELLP syndrome and/or birth. The base case analysis includes the cost of the sFlt-1/PlGF ratio test, initial visit, outpatient appointments and diagnostic procedures, anti-hypertensive medication in the outpatient setting and treatment costs associated with hospitalization. The costs of complications, namely unplanned emergency visits and neonatal intensive care admissions, are considered in a specific sensitivity analysis.
Unit costs of healthcare resources related to initial visit, outpatient management and emergency visits were taken from Portuguese official tariffs established by national legislation33. Hospitalization cost (e626,48) was inputted from a health economic study from 2012-2014 on the values of homogenous diagnostic groups (DRG) relating to PE as a primary disease (inflation not accounted for)34. The cost of anti-hypertensive medication (nifedipine) is obtained from the National Drug database considering a reimbursement of 69%35. Cost of neonatal intensive care unit admission is derived from DRG 608 (6711,50e)33. The sFlt-1/PlGF test cost was estimated as 45e. Cost inputs are presented in Table I.
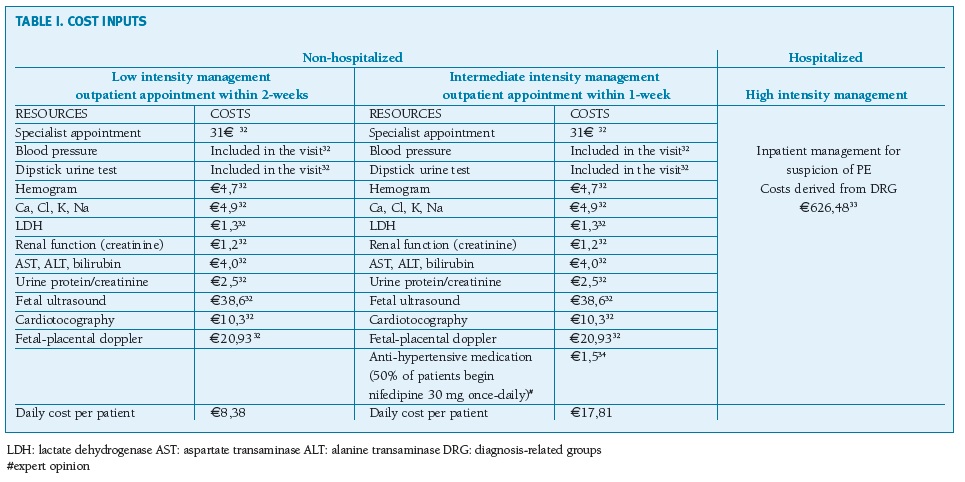
(clique para ampliar ! click to enlarge)
Sensitivity analysis
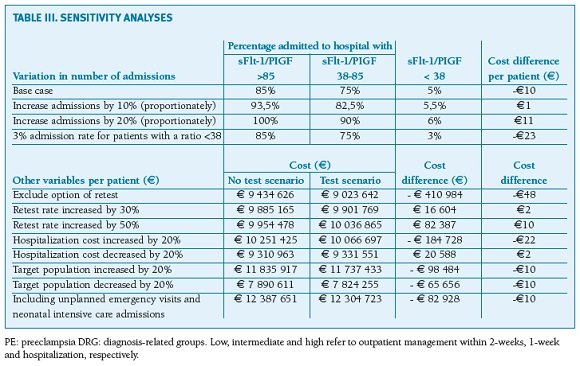
To evaluate if changes in key inputted variables yielded major deviations to the main results, six sets of sensitivity analysis were run in parallel. Specifically, the following model parameters were tested: (1) increase the admission rates of women with ratios <38, 38-85 [110] and > 85 [110] by 10% and 20%; (2) decrease the admission rate of women with ratio <38 to 3%; (3) a scenario of no retest, 30% and 50% increase on the base case; (4) vary the cost of hospitalization by ±20%; (5) vary the target population by ±20%; and (6) include costs of unplanned emergency visits and neonatal intensive care admissions.
Results
Overall, a simulated cohort of 8500 pregnant women with suspected PE, including the sFlt-1/PlGF ratio as an additive diagnostic test, yielded a positive economic effect to the SNS. In the current standard practice (no test), total costs were estimated as e9 863 264 (e1160 per patient). The key expense drivers are the treatment and management costs associated with admissions, particularly those associated with false-positive diagnoses (unnecessary admissions) representing about e3,5 million in the current practice scenario. Overall, 36.1% of women with suspicion of PE are estimated to be hospitalized (3069 women), of whom 24.6% (756 women) will develop PE. In the test scenario (initial test plus re-test) total costs sum up to e9 781 194 (e1150 per patient), representing a cost saving of e82 070 (e10 per patient) compared to standard practice (Table II). The introduction of the sFlt-1/PlGF test is estimated to reduce false-positives, thereby decreasing the rate of unnecessary hospitalizations. The hospitalization rate would decrease to 23.1% (1960 women), of whom 41.8% (819 women) would develop PE, representing fewer 1109 unnecessarily admissions per year. This generates savings of nearly e1.4 million (women without PE not hospitalized), which offsets the cost of the test and re-test itself (e552 160) and the additional expenses of following more subjects in outpatient care (e744 238).
Discussion
Overall, the financial benefit obtained by the test was consistent across a range of variations in the main parameters (Table III). Increasing the proportion of women admitted to hospital or undergoing retest, in simulation of more conservative scenarios, increases total net cost in the range of e1 to e11 per patient. Similarly, variations up to 20% in the hospitalization cost have small impact on the estimated net results. Varying the number of the target population, also has no impact on the net results.
Discussion
The syndromic nature of PE makes diagnosis and management difficult. When PE is suspected, patients are often admitted to the hospital for further monitoring/investigation and discharged only when PE is ruled out. Given the nonspecific presentation of the syndrome, many patients are unnecessarily admitted, while some cases may be missed. In both situations, physicians become vulnerable to error, patients suffer, and healthcare costs are increased.
The sFlt-1/PlGF ratio is a new immunoassay recently available in Portugal for the short-term prediction and differential diagnosis of PE. It was shown useful to rule out the disease with high negative predictive values (99.3% and 97.9% within 1 and 2 weeks, respectively) as well as to evaluate the need for hospitalization in patients presenting with signs and symptoms of PE26,27,36. To further assess the assay’s value to the healthcare system, this study modelled its impact on costs incurred by the SNS. Budget impact analysis are of most importance for helping physicians, healthcare payers and reimbursement authorities to assess affordability for the health care system, a major concern for budget holders with scarce financial resources. Our analysis quantifies the cost impact of implementing a risk stratification approach for suspected PE by integrating the sFlt-1/PlGF ratio in clinical practice. A clinical pathway was simulated based on a decision tree model where women assessed at low risk for development of the disease are discharged for outpatient management, while those at higher risk are intensively monitored or admitted.
The results predict that determining the sFlt-1/PlGF ratio is associated with a net saving of e10 per patient tested. With approximately 8500 pregnancies in Portugal each year with hypertensive disorders, total national savings could theoretically amount to e82 070 annually. This is attributed to the test’s ability to better classify patients relative to current practice, particularly its ability to reduce false positives and related unnecessary hospitalizations. It is estimated that the use of the sFlt-1/PlGF ratio allows a reduction of about 1100 unnecessary hospitalizations per year to manage the risk of PE.
The base case analysis assumes that nearly one third of the initial target population would repeat the test once, namely those women with initial negative results but with persisting or new-onset signs or symptoms. This likely reflects real-world practice - a negative result can rule out PE in the short term but not for the whole pregnancy - and therefore serial measurements may be indicated in some women29,30,37.
Sensitivity analyses of stretched scenarios demonstrated that the model was not overly sensitive to any input or assumption (cost difference per patient ranging from -e48 to e11), indicating a reasonable degree of confidence in the results.
Compared to similar economic studies from other European countries, the estimated savings in this model are modest. Vatish et al. (2016)38 showed, from a UK Health Service payer’s perspective, that implementing the sFlt-1/PlGF ratio could save £344 per patient. For the Italian system, estimated savings were up to e670 per patient39. Two main reasons explain the difference observed in our study. First, our model’s clinical inputs are more conservative. The inputted admission rates are major variables for the cost estimation and more conservative admission scenarios will ultimately lead to more expenditure. Compared to the UK study, as the thresholds for hospitalization in Portugal are likely more conservative than those recommended by NICE guidelines12, we estimated a nearly three times higher hospitalization rate among women with negative test results. Further, unlike the UK and Italian studies, in our base case we considered a reflex increase of nearly 30% in admissions of women presenting with positive ratios. This is supported by recent studies showing an increased hospitalization rate in this subgroup of women36. Second, the mean DRG tariffs for admissions of PE related disorders in the SNS are significantly down priced compared to those systems, thus reducing the potential savings associated with fewer hospitalizations. Nevertheless, results of these studies point in the same direction of a cost benefit, further supporting that the test is affordable for the SNS.
The study has certain limitations. The reliability of any budget impact model are directly related to the quality of the data used to generate it. If the clinical decision tree and estimated costs do not reflect real-world practice, results will be inaccurate. The main limitation of our analysis is that no local data on the test performance was available. Full PROGNOSIS dataset was used that includes data from several European countries other than Portugal. Although variability exists between countries, the large sample size of the study is expected to provide a good estimate of the population mean. However, the observational nature of the data limits the accuracy of some inputs. Where data was unavailable, estimates were derived according to the authors’ perception of the Portuguese reality. Sensitivity analyses were hence needed to test the impact of data inputs on model results. The model also does not account for the heterogeneity of PE management. In clinical practice the perception of "suspected PE" is highly variable and can differ from the criteria applied in PROGNOSIS, which can affect the predictive values of the test. Further, a number of clinical protocols may be in practice and criteria for hospitalization may differ among institutions and our base case. Of note, the model does not estimate the financial impact at the institution level. Real cost of a PE related admission is likely higher than the established in DRGs, meaning the savings at the institution level may be higher than those estimated in our study. Finally, the model did not account for the impact of the test on PE related adverse outcomes such as eclampsia, maternal and neonatal mortality or long term complications of prematurity. The direct and indirect costs of such complications are believed to be high40,41. Since the sFlt-1/PlGF test may help a timely identification and appropriate management of PE patients, it can decrease short and long term related adverse outcomes and associated costs. However, such potential benefits were not captured by the scope of the model. As results from ongoing and planned randomized clinical trials become available in the next few years and further insight is provided on the impact of the sFlt-1/PlGF ratio in clinical outcomes, health-economic estimations should be re-evaluated.
Conclusions
The results of this budget impact study provide favorable economic evidence about the introduction of the Elecsys sFlt-1/PlGF ratio in the SNS. Improved PE diagnosis leads to more appropriate hospital admissions and interventions and allows for a better allocation of resources. The generated savings appear to offset the costs related to the test.
REFERÊNCIAS BIBLIOGRÁFICAS
1. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol 2013;122:1122-31.
2. Magee LA, von Dadelszen P, Stones W, Mathai M. The FIGO textbook of pregnancy hypertension. London: The Global Library of Women’s Medicine. 2016. Chapter 4, 63-74.
3. James PR, Nelson-Piercy C. Management of hypertension before, during, and after pregnancy. Heart 2004;90:1499-504. [ Links ]
4. World Health Organization. Recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva: World Health Organization. 2011. [ Links ]
5. Huda SS, Freeman DJ, Nelson SM. Short- and long-term strategies for the management of hypertensive disorders of pregnancy. Expert Rev Cardiovasc Ther 2009;7:1581-94. [ Links ]
6. Póvoa AM, Costa F, Rodrigues T, Patrício B, Cardoso F. Prevalence of hypertension during pregnancy in Portugal. Hypertens Pregnancy 2008;27:279-84. [ Links ]
7. Oliveira N, Carrilho B, Carocha A, Martins AT, Cohen A, Martins I, Cruz J, Campos A. First trimester prediction of pre-eclampsia in low risk pregnancies: determining the cut-off in a portuguese group. Acta Obstet Ginecol Port. 2015;9(5):366-73. [ Links ]
8. Teixeira C, Tejera E, Martins H, Pereira AT, Costa-Pereira A, Rebelo I. First trimester aneuploidy screening program for preeclampsia prediction in a portuguese obstetric population. Obstet Gynecol Int 2014:435037. [ Links ]
9. Roberts CL, Ford JB, Algert CS, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open 2011;1:e000101. [ Links ]
10. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391-403. [ Links ]
11. Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97-104. [ Links ]
12. National Institute for Health and Care Excellence (NICE). Hypertension in pregnancy (NICE clinical guideline 107). 2011.
13. Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstet Gynecol 2001;97:261-267. [ Links ]
14. Rana S, Schnettler WT, Powe C, et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy 2013;32:189-201. [ Links ]
15. Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012;125:911-919. [ Links ]
16. Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol 2012;206:58.e1-8. [ Links ]
17. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649-658. [ Links ]
18. Staff AC, Benton SJ, von Dadelszen P, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension 2013;61:932-942. [ Links ]
19. Karumanchi SA, Epstein FH. Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of preeclampsia? Kidney Int 2007;71:959-961. [ Links ]
20. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014;10:466-480. [ Links ]
21. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672-683. [ Links ]
22. Ohkuchi A, Hirashima C, Suzuki H, et al. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res 2010;33:422-427. [ Links ]
23. Schiettecatte J, Russcher H, Anckaert E, et al. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem 2010;43:768-770. [ Links ]
24. Stepan H, Hund M, Gencay M, et al. A comparison of the diagnostic utility of the sFlt-1/PlGF ratio versus PlGF alone for the detection of preeclampsia/HELLP syndrome. Hypertens Pregnancy 2016;35:295-305. [ Links ]
25. Zeisler H, Llurba E, Chantraine F, et al. Soluble fms-Like Tyrosine Kinase-1-to-Placental Growth Factor ratio and time to delivery in women with suspected preeclampsia. Obstet Gynecol 2016;128:261-269. [ Links ]
26. Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016;374:13-22. [ Links ]
27. Zeisler H, Llurba E, Chantraine F, et al. The sFlt-1/PlGF Ratio: ruling out pre-eclampsia for up to 4 weeks and the value of retesting. Ultrasound Obstet Gynecol 2018. Accepted author manuscript.
28. Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014;63:346-352. [ Links ]
29. Stepan H, Herraiz I, Schlembach D, et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol 2015;45:241-246. [ Links ]
30. Herraiz I, Llurba E, Verlohren S, Galindo A; Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the diagnosis and prognosis of preeclampsia with the aid of the sFlt-1/ PlGF ratio in singleton pregnancies. Fetal Diagn Ther 2018;43:81-89. [ Links ]
31. Vaz-de-Macedo C, Clode N. sFlt-1/PlGF ratio as a predictor of pre-eclampsia in the second and third trimesters of pregnancy: is clinical use supported by the evidence? Acta Obstet Ginecol Port 2017;11(2):76-79. [ Links ]
32. PORDATA. INE/DGS/MS - Inquérito aos Hospitais: partos nos hospitais. Total e por tipo [online] 2018. Available from: https://www.pordata.pt/Portugal/Partos+nos+hospitais+total+e+por+tipo-1509 (accessed 27-Aug-2018). [ Links ]
33. Portaria n.º 234/2015.Ministério da Saúde. Diário da República n.º 153/2015, Série I 2015; 5516-654. Available from: http://dre.pt/pdfgratis/1989/06/13100.pdf (accessed 27-Aug-2018).
34. Rocha P. Os custos do internamento da pré-eclâmpsia em 2014. Final Report. XLIV Curso de Especialização em Administração Hospitalar. Universidade Nova de Lisboa - Escola Nacional de Saúde Pública. 2016. [ Links ]
35. Autoridade Nacional de Medicamentos e Produtos de Saúde IP - INFARMED. Base de dados de medicamentos de uso humano [online]. 2018. Available from: http://app7.infarmed.pt/infomed/inicio.php (accessed 4-Apr-2018). [ Links ]
36. Klein E, Schlembach D, Ramoni A, et al. Influence of the sFlt-1/PlGF Ratio on clinical decision-making in women with suspected preeclampsia. PLoS One 2016;11:e0156013. [ Links ]
37. Schoofs K, Grittner U, Engels T, et al. The importance of repeated measurements of the sFlt-1/PlGF ratio for the prediction of preeclampsia and intrauterine growth restriction. J Perinat Med 2014;42:61-68. [ Links ]
38. Vatish M, Strunz-McKendry T, Hund M, Allegranza D, Wolf C, Smare C. sFlt-1/PlGF ratio test for pre-eclampsia: an economic assessment for the UK. Ultrasound Obstet Gynecol 2016;48:765-771. [ Links ]
39. Frusca T, Gervasi MT, Paolini D, Dionisi M, Ferre F, Cetin I. Budget impact analysis of sFlt-1/PlGF ratio as prediction test in Italian women with suspected preeclampsia. J Matern Fetal Neonatal Med 2017;30:2166-2173. [ Links ]
40. McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008;156:918-930. [ Links ]
41. Sibai BM. Caring for women with hypertension in pregnancy. JAMA 2007;298:1566-1568. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Nelson Lopes
E-Mail: nelson.lopes@roche.com
Recebido em: 25/06/2018
Aceite para publicação: 21/09/2018