Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Obstétrica e Ginecológica Portuguesa
versão impressa ISSN 1646-5830
Acta Obstet Ginecol Port vol.13 no.2 Coimbra jun. 2019
CASE REPORT/CASO CLÍNICO
Primary fetal pleural effusion - a case of successful prenatal intervention
Derrame pleural fetal primário - um caso de sucesso da terapêutica pré-natal
Beatriz Bettencourt Silva1, Dânia Ferreira2, Miguel Branco3, Elsa Pereira4, Adosinda Rosmaninho4
Serviço de Ginecologia e Obstetrícia, Hospital da Senhora da Oliveira - Guimarães, E. P. E.
Maternidade Bissaya Barreto, Centro Hospitalar e Universitário de Coimbra, E. P. E.
1 Médica Interna de Formação Específica de Ginecologia e Obstetrícia, Hospital da Senhora da Oliveira - Guimarães
2 Assistente Hospitalar de Ginecologia e Obstetrícia, Hospital da Senhora da Oliveira - Guimarães
3 Assistente Hospitalar Graduado de Ginecologia e Obstetrícia, Maternidade Bissaya Barreto, Centro Hospitalar e Universitário de Coimbra
4 Assistente Hospitalar Graduada de Ginecologia e Obstetrícia, Hospital da Senhora da Oliveira - Guimarães
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Fetal pleural effusion is a rare condition whose prognosis is highly variable. Most primary cases are chylothorax and prenatal interventions can significantly alter its natural course. Some authors defend thoracoamniotic shunting as the preferred intervention but in few cases the effusion may resolve or stabilize after a single transabdominal thoracentesis. The authors present a case of primary bilateral pleural effusion identified at the 31st gestational week. A thoracentesis was performed at the 32nd week. A little effusion was maintained, with no reaccumulation. The newborn had an appropriate respiratory response at delivery and no thoracentesis was required in the neonatal period.
Keywords: Primary hydrothorax; Prenatal management; Therapeutic thoracentesis.
Introduction
Fetal pleural effusion (also referred as fetal hydrothorax) is an accumulation of fluid in the pleural space, which can present either uni- or bilaterally. It is a rare condition that affects 1 in 10 000 to 15 000 pregnancies, being more frequent in males than females (2:1)1. Recent data suggests it can be more common than originally described2. It may occur as an isolated finding or in association with several conditions, namely as part of nonimmune hydrops or severe anemia. There is a well-established association with chromosomal anomalies, mainly Down syndrome, and genetic syndromes, including Noonan’s syndrome, Opitz-Frias hypertelorism hypospadias syndrome and Caffey’s cortical hyperostosis1. Congenital infections, cystic hygroma, congenital pulmonary lymphangiectasias and cardiac malformations can also be associated with this condition1,3.
Bilateral effusion is the most common clinical manifestation2-4. The median gestational age at diagnosis and at delivery is around 30-32 weeks and 34-35 weeks, respectively1,3-5. Most primary pleural effusions are lymph accumulation (chylothorax) and it probably results from abnormalities of the lymphatics vessels1,3,6.
The clinical course of this condition is variable and difficult to predict. It ranges from spontaneous resolution to progressive increase and development of hydrops with high risk of preterm delivery or fetal death1,2. Perinatal overall mortality between 22 and 53% has been reported, reaching 75% in untreated pleural effusions associated with hydrops1,3-5.
The available evidence about prenatal management is based essentially on retrospective studies of case series or case reports. Although there is no consensual recommendations, recent data supports that prenatal intervention can significantly alter the natural course of primary pleural effusions3-5.
The authors describe a case of primary bilateral pleural effusion identified at the 31st gestational week which had a good neonatal outcome after a single prenatal thoracentesis. The therapeutic option and the facts that eventually predict a good prognosis will be briefly discussed.
CASE REPORT
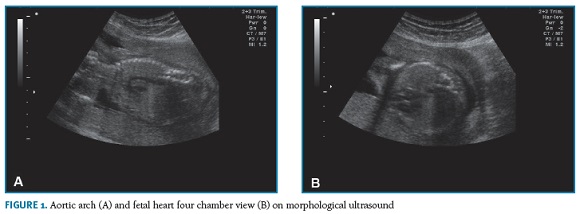
A 44-year-old patient, gravida 5 para 1 (1 vaginal delivery, 3 first trimester spontaneous abortions after age 40) with no relevant medical history, was referred to our obstetric department by a first trimester combined screening with high risk for trisomy 21 (1:30). No structural anomaly was detected and nuchal translucency was below 95th percentile in the first trimester ultrasound, which was performed in another hospital. Despite the advanced gestational age at the first appointment (19th gestational week), she underwent an amniocentesis whose result revealed a normal male karyotype. Due to maternal age, she maintained pregnancy surveillance in our department. At the 24th week, gestational diabetes was diagnosed. Blood glucose values were controlled with dietary plan and insulin injections. Morphological ultrasound (Figura 1-A,B) and fetal echocardiogram, at the 21st and 27th weeks respectively, were unremarkable. The fetal echocardiogram was requested as an insurance, considering the maternal age and the early onset of insulin to control the glucose blood values.
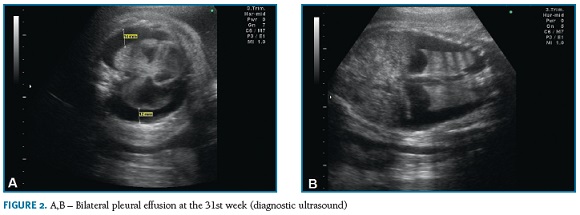
On the third trimester ultrasound, performed at the 31st week, bilateral pleural effusion with lung atele- ctasis (Figura 2-A,B) and polyhydramnios (amniotic fluid index of 27,8 cm) were identified. No other structural abnormalities were detected. There were no signs of fetal anemia (peak systolic velocity in the middle cerebral artery was normal) or hydrops, namely pericardium effusion, ascites, subcutaneous edema or placentomegaly. Fetal biometry was compatible with gestational age and cervical length evaluation was normal (35mm).
Antibody screening, including red cell antibodies, and screening for congenital infections (toxoplasmosis, syphilis, rubella, cytomegalovirus and herpes simplex virus) were negative.
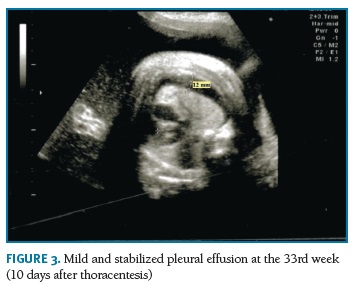
A transabdominal thoracentesis under ultrasound control was performed at the 32nd week, using a 20-gauge spinal needle. A turbid fluid was removed, which composition suggested the diagnosis of chylothorax (no erythrocytes, total white blood cells count of 5.20x103/ìL, with lymphocyte proportion of 97%). Serial ultrasound evaluations confirmed the success of the treatment and revealed only a little and stabilized bilateral effusion (Figura 3). Polyhydramnios was maintained. Fetal growth assessment was normal, with estimated fetal weight between the 60th and 70th percentiles. After the intervention antenatal corticosteroid therapy for pulmonary maturation was performed with intramuscular injection of 12 mg of betamethasone renewed 24 hours later.
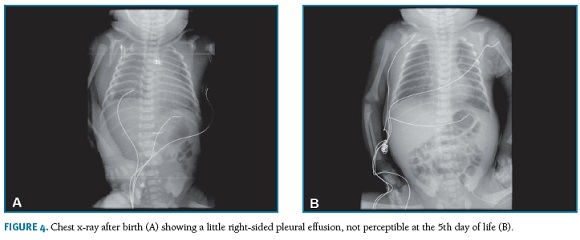
At the 33rd week, the pregnant woman was admitted with threatened preterm labor. She went into spontaneous labor and a cesarean section was performed at the 34th week (12 days after thoracocentesis) for non-reassuring fetal status. The newborn weighing 2632 grams required transient ventilator support with appropriate respiratory response (Apgar score of 6, 9 and 10 at 1, 5 and 10 minutes, respectively). The chest x-ray revealed a little right-sided pleural effusion that spontaneously resolved (Figura 4-A,B), with no need of thoracentesis in the neonatal period.
During the first 2 years of life, the infant had three episodes of mild acute bronchiolitis. Four years post-partum follow-up evaluation confirmed he was a healthy child and had a normal development for his age.
Discussion
The authors present a case of successful prenatal management of a primary pleural effusion identified at the 31st gestational week.
On the etiological investigation we did not offer screening of Noonan’s syndrome. However, there is growing evidence that late presenting pleural effusions (after fetal viability) are commonly associated with that condition, so genetic testing should be considered for those cases2.
The fluid removed by transabdominal thoracentesis suggested the diagnosis of chylothorax. In newborns the diagnosis of chylothorax is confirmed by thoracentesis when the fluid contains triglyceride levels > 1.1 mmol/L (with oral fat intake) and total white blood cells count > 1000/ìL, with lymphocyte proportion >80%1,3,6. Although the normal mean percentage of lymphocytes in the fetal blood is usually >80%, several authors adapt this criteria to confirm the diagnosis in utero with transabdominal thoracentesis3,5-7.
There is no consensus about prenatal management of primary pleural effusions. Some authors had proposed therapeutic recommendations, particularly in patients for whom a diagnosis is made before or at 34 weeks1,6. Prenatal management includes serial ultrasounds, transabdominal thoracentesis, thoracoamniotic shunt placement and, rarely, in utero pleurodesis1,8. Severe pleural effusions can compromise normal lung maturation leading to pulmonary hypoplasia with a high rate of perinatal mortality. So, the main goal of those interventions is to prevent pulmonary hypoplasia and facilitate the respiratory management in the delivery room and neonatal period. Recent studies show that prenatal therapy significantly improves survival of newborns, particularly in the setting of fetal hydrops3-5. Some authors defend thoracoamniotic shunting as the preferred intervention since it provides a permanent drainage of the pleural effusion with a single procedure. There is growing evidence that it is the most effective intervention, especially in cases of fetal hydrops or rapid effusion progression1,4,5,9. The most common complication is shunt migration or obstruction, which may occur in more than 20% of the procedures1,4. On the other hand, some authors choose thoracentesis at first, because it stablish the diagnosis, is technically easier to preform and is potentially therapeutic. Its main limitation is the risk of rapid effusion reaccumulation, occurring generally within 1 hour to 10 days 1,10. In few cases the effusion may resolve or stabilize after a single thoracentesis, especially in non-hydropic fetuses1,4,5,10. However, there are studies reporting a high rate of repeat intervention after the first thoracentesis or disappointing survival rates after an antepartum thoracentesis to facilitate neonatal resuscitation9,10. It is important to note that those series included a high proportion of hydropic fetuses. Both thoracentesis and thoracoamniotic shunting appear to be well tolerated and the global incidence of complications is low. Nevertheless, its potential benefits should be balanced against the risk of prematurity and premature rupture of membranes, which remain the greater concerns4.
Despite prenatal management, after delivery some newborns remain asymptomatic or experience only mild respiratory distress, while others need immediate resuscitation, possibly requiring urgent pleural drainage1,3. Several factors have been associated with a poor prognosis after prenatal detection of pleural effusions, including the presence of a secondary cause, structural anomalies, fetal hydrops, low birth weight, preterm birth less than 34 weeks, multiple or large effusions4,8. On the other hand, spontaneous resolution seems to be more common in primary unilateral cases diagnosed in the second trimester1,2,10.
In the present case, important secondary causes were excluded even before the diagnosis. The performance of amniocentesis ruled out the possible association with chromosomal aneuploidies, namely Down syndrome, which are described in 11 to 14% of congenital pleural effusions2,4,9. The association with congenital heart defects seems to be around 15 to 20%4,9. A normal morphologic ultrasound and fetal echocardiogram were against this hypothesis. After the diagnosis, autoimmune or infectious etiology were also excluded. Furthermore, the absence of hydrops or other structural anomalies, the moderate severity of the pleural effusion at diagnosis and the appropriate birth weight for the gestational age seem to have promoted the positive neonatal outcome4,8,10. The corticosteroid therapy completed more than 24 hours before the delivery may have been an important contributor for the good respiratory response. In case series of primary pleural effusions, polyhydramnios seems to be a relatively frequent finding and has no stablished prognostic impact2,7,10. On the other hand, in this case the pregnant woman was diagnosed with gestational diabetes, which represents a well stablish etiology of polyhydramnios11.
During the first 2 years of life, the infant had three episodes of acute bronchiolitis, one of them with hospitalization. Interestingly, a retrospective study of congenital chylothorax with a minimum 3-years postpartum follow-up reported that 4 of the 15 survivors were diagnosed as having asthma in early infancy, placing the hypothesis that even the cases with no neonatal relapse can have subsequent pulmonary function impairment9. However, all of those patients were also born prematurely and had recurrent respiratory infections. In our case, the subsequent diagnostic investigation reported normal pulmonary function tests. Long-term follow-up studies also suggested that most of the survivors diagnosed with primary chylothorax remain asymptomatic, with an age-appropriate development. Neurocognitive impairment seems to be related to associated anomalies or extreme prematurity8,9.
In conclusion, prenatal diagnosis of fetal pleural effusion implies a thorough etiological research and serial ultrasounds to assess its evolution and possible associated anomalies. Although this condition is still often associated with poor outcomes, advances in prenatal medicine are improving the prognosis of these patients and nowadays most of them are likely to survive. The optimal strategy for fetal intervention has not been fully elucidated. According to the actual evidence, a case-by-case evaluation based on time of diagnosis, effusion severity and prognostic factors seems to be the most reasonable approach.
REFERENCES
1. Rustico MA, Lanna M, Coviello D, Smoleniec J, Nicolini U. Fetal pleural effusion. Prenat Diagn 2007;27:793-799. [ Links ]
2. Wellesley D, Howe DT. Diagnosis and outcome in nonhydropic fetal pleural effusions. Prenat Diagn 2018.
3. Lee CJ, Tsao PN, Chen CY, Hsieh WS, Liou JY, Chou HC. Prenatal Therapy Improves the Survival of Premature Infants with Congenital Chylothorax. Pediatr Neonatol 2016;57:127-132. [ Links ]
4. Mon RA, Treadwell MC, Berman DR, et al. Outcomes of fetuses with primary hydrothorax that undergo prenatal intervention (prenatal intervention for hydrothorax). J Surg Res 2018;221:121-127. [ Links ]
5. Dorsi M, Giuseppi A, Lesage F, et al. Prenatal factors associated with neonatal survival of infants with congenital chylothorax. J Perinatol 2018;38:31-34. [ Links ]
6. Rocha G, Guerra P, Azevedo I, Guimaraes H. [Chylothorax in the fetus and the neonate-guidelines for treatment]. Rev Port Pneumol 2007;13:377-381. [ Links ]
7. Echeverria Lecuona J, Benito A, Arena Ansotegui J, Collado Espiga V, Rey Otero A, Paisan Grisolia L. [Congenital chylothorax]. An Esp Pediatr 1998;49:161-164. [ Links ]
8. Chiang MC. Congenital Chylothorax: Antenatal Intervention, Survival, and Outcome. Pediatr Neonatol 2016;57:85-86. [ Links ]
9. Caserio S, Gallego C, Martin P, Moral MT, Pallas CR, Galindo A. Congenital chylothorax: from foetal life to adolescence. Acta Paediatr 2010;99:1571-1577. [ Links ]
10. Petersen S, Kaur R, Thomas JT, Cincotta R, Gardener G. The outcome of isolated primary fetal hydrothorax: a 10-year review from a tertiary center. Fetal Diagn Ther 2013;34:69-76. [ Links ]
11. Hamza A, Herr D, Solomayer EF, Meyberg-Solomayer G. Polyhydramnios: Causes, Diagnosis and Therapy. Geburtshilfe Frauenheilkd 2013;73:1241-1246. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Beatriz Bettencourt Silva
Hospital Senhora da Oliveira - Guimarães, E.P.E, Serviço de Ginecologia e Obstetrícia
Rua dos Cutileiros, Creixomil 4835-044 Guimarães, Portugal. Tel. +351 253 540 330
E-mail: beatrizbettsilva@gmail.com
Recebido em: 21/11/2018
Aceite para publicação: 03/05/2019