Introduction
Ulipristal acetate (UPA) has been available in Europe since 2012 for the treatment of moderate to severe symptoms of uterine fibroids in reproductive age women. According to data from the European Medicines Agency (EMA), it is estimated that more than 765,000 women have been treated up to February 20181.
In February 2018, the Pharmacovigilance Risk Assessment Committee (PRAC) of the EMA issued a temporary recommendation advising that new courses of treatment with UPA should not be initiated. The local regulatory entity also transmitted this and further recommended monitoring the liver function of patients currently under treatment. UPA should be suspended if transaminases levels exceed twice the maximum limit2.
The Portuguese Society of Gynaecology decided to survey its members’ experience with UPA using an online questionnaire, in order to contribute to better knowledge about its use in Portuguese women, particularly regarding liver function.
Methods
This cross-sectional study was conducted among members of the Portuguese Society of Gynaecology between April and May 2018. All members of the Portuguese Society of Gynaecology have allowed access to their e-mail contact for Society surveys. The Board of the Portuguese Society of Gynaecology gave permission for the application of the questionnaire.
Ethical approval was not necessary as the members of the Portuguese Society of Gynaecology were freely invited to complete the questionnaire and gave their consent to access the email address.
The entire online database represents a total of 783 members that receive the survey, residents and specialists in gynaecology, working in public and private practice in Portugal. The questionnaire was administered anonymously using a link received by e-mail to an online survey platform.
The study instrument had twenty-two questions and was restricted to questions about the prescription of UPA since January 2018, either for the continuation or beginning of treatment.
The first part consisted of questions assessing the number of patients treated and the previously available assessment of hepatic function, with particular emphasis on transaminases, aspartate transaminase (AST) and alanine transaminase (ALT), gamma-glutamyltransferase (GGT), bilirubin (BRB) and alkaline phosphatase (ALP).
The second part addressed the assessment of hepatic function after the beginning of treatment. The survey inquired about clinical and laboratory findings, namely increase in levels of AST, ALT, BRB of twice the normal upper limit and three times the normal upper limit. The study of other laboratory tests was also requested as well as prior hepatic diseases of other aetiologies.
The third part assessed the need to suspend treatment, the clinical course of hepatic dysfunction and the subsequent treatment of uterine fibroids.
Results
Sixty-two gynaecologists answered the survey, a response rate ranged 7,9%. Considering the responders, 48 had prescribed UPA for new or continued treatment after 1st January 2018. During this period, 199 women were treated.
The physicians who prescribed the drug reported that liver function had been assessed before treatment in 19 cases. In three of these cases alterations were detected in liver function (elevation of transaminases in two cases and GGT in one case) prior to treatment.
According to the responders, the evaluation of hepatic function was performed in 96 women after UPA start and in 63 the assessment of liver function included not only transaminases but also bilirubin.
In five cases, described in Table I, were detected elevation of transaminases; in three cases the blood concentration level did not double but in two cases there was an increase greater than threefold.
Table I Cases of upa use for uterine fibroids with alteration in liver function
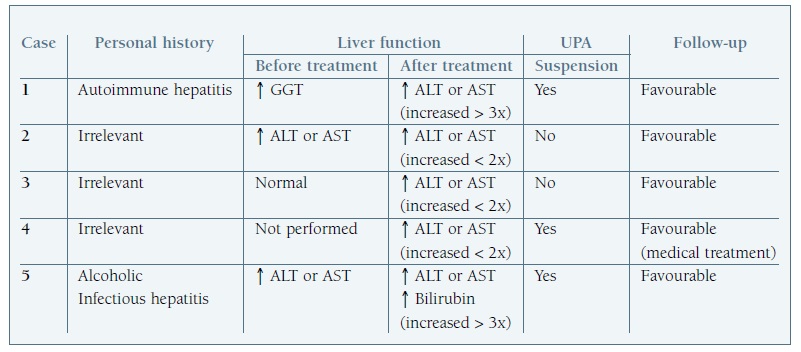
↑: increase; x: times; aspartate transaminase (AST); alanine transaminase (ALT); gamma-glutamyltransferase (GGT).
In the cases in which hepatic function was altered after treatment, complementary studies were performed in three women and hypoalbuminemia and abnormalities in hepatic ultrasound were detected.
Considering the five women diagnosed with hepatic impairment: there was a history of liver disease in two cases (alcoholic, infectious liver disease and autoimmune hepatitis); treatment with UPA was suspended in three of these patients (and one patient continued medical treatment with a hormonal contraceptive); other two cases continued treatment without other adverse events.
In all cases, a favourable evolution of liver function abnormalities diagnosed during monitoring was described.
Discussion
The incidence of drug-induced liver injury (DILI) is between 10 and 15 per 10000 to 100000 exposed3. DILI accounts for 10% of cases of acute hepatitis and is the most frequent cause of acute liver failure in the United States4. This is the first reason to withdraw drugs from the market. Hepatic toxicity may not be detected in clinical trials prior to drug approval because many trials include fewer than 3000 participants and adverse events with an incidence lower than 1 in 10000 are rare5.
More than 1000 products, medicines and herbal products have been associated with liver toxicity6. Many of these are currently used in clinical practice, particularly antibiotics such as amoxicillin/clavulanic acid and erythromycin; antifungals such as imidazole, fluconazole and itraconazole; antithyroid drugs such as propylthiouracil and methimazole; and also oral hormonal contraceptives, which are among the most often prescribed by gynaecologists. The mechanisms of toxic hepatitis by drugs are not yet fully understood and include hepatocellular and extracellular mechanisms with lesion of bile duct epithelium3.
There are two main groups of reactions to drugs: direct liver damage and autoimmune response. The intrinsic or predictable reactions cause lesions that are reproducible and dose dependent. The injury may be due to the drug itself or to a metabolite. An example of this type of toxicity is that caused by paracetamol in high doses. Idiosyncratic reactions may be subdivided into hypersensitivity or immunoallergic or those that are metabolic/idiosyncratic. In these cases the response is variable and can occur at different times and in a minority of patients7.
UPA was marketed in Europe and in Portugal in 2012 and was previously tested in several pre-clinical animal studies and phase I, II and III trials8. Animal studies demonstrated no hepatic toxicity and no molecular similarities to compounds that have been associated with DILI8. It was in the post-marketing period that severe hepatic lesions were reported, however with a variable clinical pattern, namely onset of symptoms from within a few days to months after the first dose. The association with underlying chronic liver disease and absence of complete information on concomitant medications and co-morbidities does not allow a cause-effect relationship to be established between the drug and these adverse events. However, as such an association cannot be completely ruled out, this justifies the recommendations focusing on the need for close monitoring before the onset and during the treatment with UPA9.
Potential confounders are pointed to this study, namely the voluntary participation of the society members. Several aspects of clinical data were not included as the aim was to describe clinical experience and liver function alterations related to treatment. This study can have potential bias, considering it is an observational study conducted by a scientific society to the members by e-mail. Besides this fact the unique source of clinical data is the doctor report. We also recognize a low response rate, but we aimed to analyse the clinical experience and the severity of adverse outcomes considering the liver function.
Our survey provided information about 199 patients who received UPA in Portugal. Of this group, 2.5% (five cases) presented elevated liver enzymes after onset of treatment, but in only 1% (two cases) with clinical relevance. It should be noted that in both cases there was a previous history of liver disease, what emphasizes that UPA should not be considered in patients with previous liver disease. All cases had a favourable evolution. In cases with mild elevation of transaminases, the continuation of UPA did not worse laboratory data and no serious adverse effect were reported. More severe enzyme levels did not continued treatment.
UPA is an effective drug but the relationship between UPA and rare hepatic lesions led to temporary suspension in 202010. In the future, the use of UPA will probably be limited to selected clinical sceneries, namely surgical contraindication, fibroid embolization and surgical fails11. The results of this investigation reinforce the need for careful and systematic collection of the clinical data of women treated with UPA and strict monitoring of hepatic function according with authorities’ recommendations of 2021. EMA restricted the use of UPA for intermittent treatment of moderate to severe symptoms of uterine fibroids in premenopausal women, when other options are not adequate or failed.















