Introduction
The novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly across the globe with continuous increasing numbers. The rate of pregnant women infected with this pathogen is also increasing and concerns about its impact on both fetal and maternal wellbeing have risen worldwide. Prevalence reports published so far are quite heterogeneous, with some centers describing infection rates as low as 0.47%1 and others as high as 15.4%.2 In Portugal, a prevalence of 6% has been described. (3
Pregnancy is associated with physiological and immunological changes, making pregnant women an unique and vulnerable group for many infectious diseases, usually with a more aggressive presentation and negative impact on maternal, obstetrical and neonatal outcomes. (4 Early data suggested that pregnant women did not have greater risk of getting infected by SARS-CoV-2 compared to the general population5 nor were prone to have more severe disease or need of admission to an intensive care unit (ICU). (6), (7), (8), (9), (10), (11 However, recent evidence describing the contrary has been published. (12), (13
Similarly to non-pregnant population, most pregnant women are asymptomatic2), (14), (15 and, when symptoms develop, the most frequent clinical presentation includes fever, cough, malaise/fatigue, (7), (16 dyspnea and lymphopenia. (8), (10), (17 Though pneumonia has been described, severe respiratory consequences are rare7,17 and, in most studies, the mortality rate seems to be similar to non-pregnant population. (9), (12), (18
Regarding pregnancy outcomes, there are some reports stating rates of preterm deliveries as high as 47%19, most of them iatrogenic due to deterioration of maternal condition or fetal distress. (8 To date, there is no evidence that correlates spontaneous preterm labor with SARS-CoV-2 infection. (10
Congenital infection by SARS-CoV-2 is a major concern and a lot of controversy exists regarding the hazard of vertical transmission. The first case report describing transplacentar transmission, with the detection of SARS-CoV-2 genes in amniotic fluid, placental tissue and cord blood, was described in July 2020. (20 Other cases in the literature describing possible vertical transmission have been reported16), (21), (23 and, to the best of our knowledge, most published data show reassuring fetal/neonatal outcomes. (16), (20), (23
The knowledge on the clinical course and impact of COVID-19 in pregnancy is still limited and there is no robust scientific evidence on the obstetric management of these patients. Therefore, our aim was to describe the clinical characteristics and outcomes of pregnant women infected with SARS-CoV-2 in a tertiary Portuguese hospital. Data analysis can be used to better understand and manage pregnant women during this pandemic.
Methods
We performed a single-center, retrospective, cohort study of pregnant women infected with SARS-CoV-2 between March 30 and July 31 of 2020 in a tertiary referral center for obstetrical care in Lisbon with over 2700 deliveries per year and a cesarean delivery rate of 33%.
We included all pregnant and immediately postpartum women with a positive real-time reverse transcription polymerase chain reaction (RT-PCR) test result for SARS-CoV-2 with antenatal care or delivery at our center.
The test was performed to all pregnant women who were admitted to the labor and delivery ward. If symptoms developed during the hospital stay, the test was repeated. Additionally, pregnant women observed in outpatient clinics or in the emergency department with COVID-19 symptoms or history of potential exposure also performed the test. Newborns of SARS-CoV-2 infected mothers were also tested. All RT-PCR tests were performed by oro and nasopharyngeal swabs.
The study was approved by the local ethics committee (registry ID number: 74/2020) and patient consent was obtained prior to the study.
We reviewed the electronic medical records of the mother and neonate. De-identified data was recorded and stored in a standardized computer spreadsheet. Data collected included demographics, medical and obstetrical history, symptoms at presentation, ICU admission, gestational age and mode of delivery, pregnancy complications and outcomes, RT-PCR test results for SARS-CoV-2 in newborns and neonatal outcomes.
Statistical analysis was carried out using IBM SPSS® version 26.0 (Statistical Package for the Social Sciences®, Inc., Chicago, IL) for MAC. Continuous variables are presented as median and interquartile range (IQR) and categorical variables in numbers (n) and percentages. Comparisons between groups were made using Chi-square or Fisher’s exact test for categorical variables and Mann-Whitney test for continuous variables. All analyses were considered statistically significant if a two-tailed p-value <0.05 was observed.
Results
Seventy-five pregnant women with confirmed SARS-CoV-2 infection were identified. Of these, 66.7% were diagnosed in the 3rd trimester of pregnancy. The median age was 28 years (IQR 6) and 74.7% were non-caucasian. Fifty-five percent were multiparous, and all had singleton pregnancies. Obesity was present in 20% (Table I).
Table I Demographic, clinical and obstetrical characteristics of pregnant women with SARS-COV-2 infection
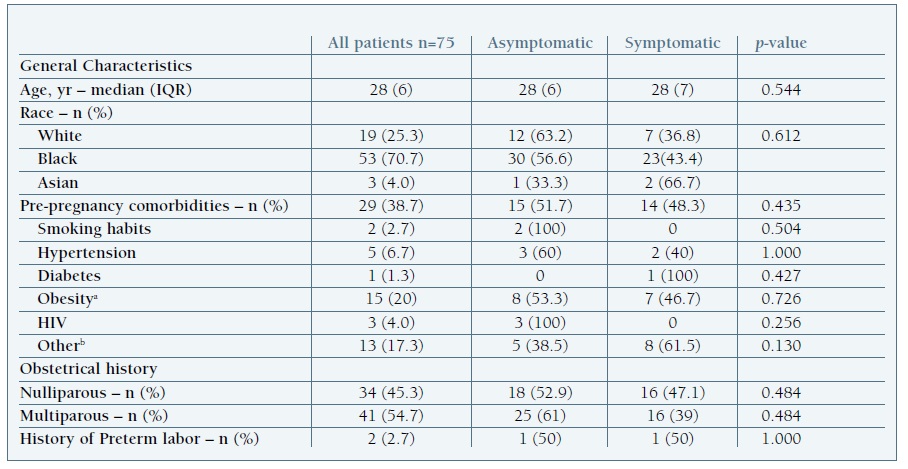
HIV, human immunodeficiency virus; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; yr, years. a Obesity was defined as Body Mass Index ≥ 30 Kg/m2 b Other maternal comorbidities included emoglobinopathies (n=3), chronic hepatitis B (n=2), asthma (n=2), hypothyroidism (n=1), previous ischemic stroke (n=1), depressive disorder (n=1), thrombophilia (n=1), previous pulmonary tuberculosis (n=1) and migraine (n=1).
The majority of women were asymptomatic (57.3%) and tested upon hospital admission for labor or related to pregnancy complications. Five patients became symptomatic postpartum, 4 of them with an initial negative test for SARS-CoV-2. Among the 32 symptomatic women, most developed mild symptoms (Table II) including fever (34.4%), cough (37.7%) and headache (34.4%); other symptoms such as ageusia/anosmia (31.3%) and myalgia (31.3%) were also commonly described. Lymphopenia was present in 13 women, 10 of which were symptomatic (p<0.001).
Table II Clinical presentation of SARS-COV-2 infection
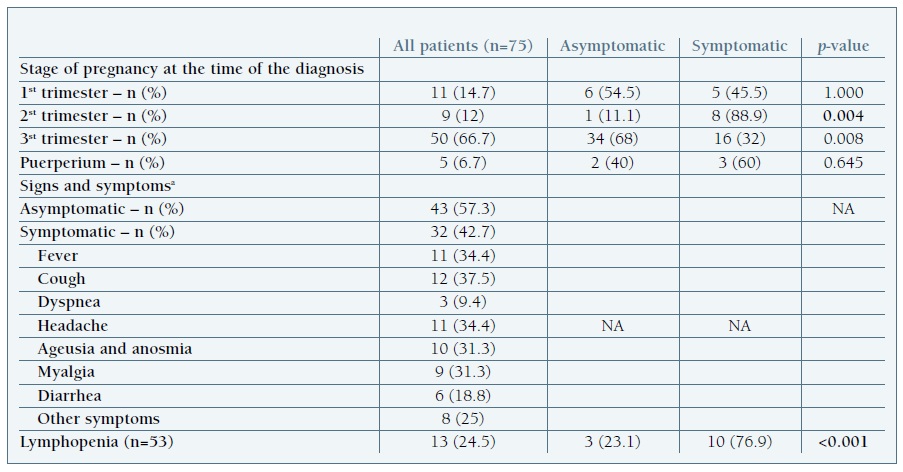
SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.a The signs and symptoms listed include those reported before admission and during hospitalization.
During the period of the study, only two cases of pneumonia were reported. Both women were nulliparous and 34 weeks of pregnancy. The first case occurred in a 26-year-old asymptomatic woman, with no previous comorbidities, admitted to the obstetric unit due to hydramnios and myoma praevia. During the hospital stay she developed cough, anosmia, fever and shortness of breath, with partial respiratory failure. A cesarean section was performed due to maternal clinical deterioration and the woman was transferred to the ICU for surveillance and non-invasive respiratory support. The second case of pneumonia occurred in a 40-year-old obese woman admitted due to fetal growth restriction and nonsevere pre-eclampsia. She presented with fever, headache and shortness of breath and, due to a sudden aggravation of her ventilatory status, a cesarean section was performed. Maternal status improved significantly after delivery, without the need for ventilatory support.
No maternal deaths were registered in the period of the study.
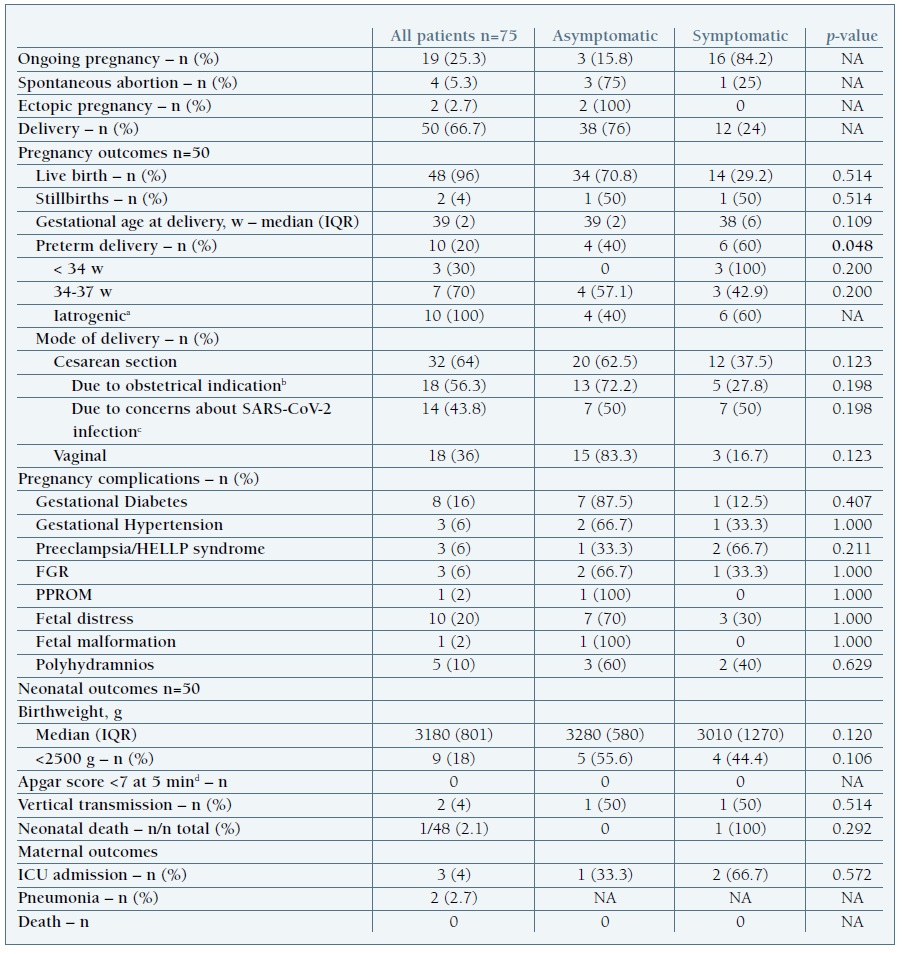
Out of the 811 women that delivered in our center during study period, 50 were infected with SARS-CoV-2 (6.2%) (Table III). The rate of cesarean section was 64% (n=32), 14 due to maternal SARS-CoV-2 infection, 10 due to fetal distress and 8 related to other obstetrical indications.
FGR, fetal growth restriction; g, grams; HELLP, hemolysis, elevated liver enzymes, low plaque count; IQR, interquartile range; min, minutes; PPROM, preterm premature rupture of membranes; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; w, weeks. a Indications for iatrogenic preterm delivery included fetal distress (n=3), fetal demise (n=2), preeclampsia/HELLP syndrome (n=2), COVID-19 pneumonia (n=2) and preterm premature rupture of membranes (n=1). b Indications for cesarean delivery included HIV infection, fetal distress, preeclampsia/HELLP syndrome, active phase arrest, mal presentation, macrosomia and previous uterine surgery. c Including two cases due to severe maternal disease; the remaining were due to concerns about intrapartum transmission. d After excluding the 2 cases of stillbirth
Of the 48 livebirths, 8 were preterm deliveries, all iatrogenic. Preterm birth was more frequent in symptomatic women (p=0.048). When analyzing specific symptoms, blood-work results and stage of disease, fever (p<0.001), dyspnea (p=0.037), diarrhea (p=0.022), lymphopenia (p=0.025) and maternal pneumonia (p=0.037) were significantly associated with premature delivery. Maternal lymphopenia was also associated with fetal distress, although without reaching statistical significance (p=0.054).
During the study period, there were 2 intrauterine fetal deaths. One occurred at 28 weeks of gestation in a pregnant patient with mild disease; the pathology report showed signs of fetal pneumonia, although pulmonary tissue analysis was negative for SARS-CoV-2. Placenta histology showed chorioamnionitis and villitis, probably secondary to a bacterial infection. The second stillborn presented at 34 weeks of gestation with severe growth restriction, pleural effusion, ascites and oligohydramnios. The fetus autopsy showed generalized oedema and hemorrhagic suffusions, with no fetal malformations. Pathology analysis of fetal pulmonary tissue was positive for SARS-CoV-2 infection22. Placenta histology revealed multiple areas of ischemia and the immunohistochemistry identified an overexpression of the protein associated with SARS-CoV-2 virus.
All newborns were tested for SARS-CoV-2 immediately after delivery and at 24-48 hours of life. One neonate born at 34 weeks of gestation due to maternal clinical deterioration tested positive for SARS-CoV-2 in neonatal blood sample collected immediately after birth and developed severe disease. There was one neonatal death due to complications related to extreme prematurity after a preterm delivery at 28 weeks in a woman with severe preeclampsia.
At the end of the study period 19 women had ongoing pregnancies (Table III).
Discussion
The first pregnant woman with COVID-19 in our institution was diagnosed on March 30, 2020. The patient developed symptoms on the 1st day postpartum. After that, and due to the increasing incidence of COVID-19 cases in Lisbon, universal screening for SARS-CoV-2 was implemented to all admissions to the labor and delivery ward in our center.
Pregnant women are included in one of the age groups where more cases of COVID-19 have been identified. (23 During the time of the study, the prevalence of SARS-CoV-2 at delivery was 6.2%, similarly to another hospital in Northern Portugal3 but significantly higher than the prevalence described in another central hospital in Lisbon1 and in other American and European studies. (14), (15), (25), (26), (27 The different testing policies adopted by each hospital (universal screening vs diagnostic testing) and publication at different times of the pandemic26 may contribute to these findings. In addition, the population covered by our center includes a high number of people with a low socioeconomic status, whose housing conditions and social behaviors may increase the risk of virus transmission.
In our cohort, a disproportionate number of black women were affected by SARS-CoV-2. Ethnic disparities in incidence and outcomes have also been reported in other series and among non-pregnant populations with COVID-19. (28), (29), (30 However, since our hospital’s referral area includes a high rate of black citizens, a selection bias has to be considered.
We also found that most women were diagnosed in the third trimester, in comparison to earlier stages of pregnancy. This might reflect the effect of the universal screening at admission, since most women were asymptomatic and admitted pre-partum.
As previously said, our patients were mostly asymptomatic at time of the diagnosis, similar to what has been reported in other studies, (1), (2), (14), (15), (26 which reinforces the need for universal testing. Like in the non-pregnant population, (24 the most reported symptoms were cough, fever and headache, which suggests that pregnant women portray a similar clinical presentation to the general population. Severe disease was uncommon, similarly to the findings in other studies in China and in the UK, (10), (16 with COVID-19 pneumonia being diagnosed in only 2 patients.
Blood work results were available in 53 patients. Lymphopenia was present in 25%, mainly in those with symptoms (p<0.001). According to recent evidence, lymphopenia may be an indicator of the severity of disease and need for hospitalization in COVID-19 patients. (31), (32 Although this was not the case in our cohort, pregnant women with low lymphocyte counts were more likely to have a preterm delivery (p=0.025) and there was a tendency towards a higher rate of fetal distress (p=0.054). Similarly, fetal distress has been pointed out as the most common adverse perinatal outcome of other coronavirus infections. (33 In addition, 54% of the SARS-CoV-2 positive women in our cohort with lymphopenia needed a cesarean delivery (either pre-term or term). We hypothesize that lymphopenia may be a marker of possible worse maternal and fetal outcomes; however, this hypothesis is limited due to the retrospective nature of the study and a selection bias cannot be excluded.
Viral pneumonia in pregnancy has been associated with an increased risk of preterm delivery, (34), (35 low birth weight35 and perinatal mortality. (35), (36 Accordingly, our findings show an association between COVID-19 pneumonia and premature birth (p=0.037), similar to what has been previously described. (33), (37
Preterm delivery was also common in patients without pneumonia, especially in symptomatic women (p=0.048). All premature births were iatrogenic, mostly due to fetal distress. The absence of spontaneous preterm delivery in our cohort is consistent with published data. (10
During the study period, there was a much higher rate of caesarian deliveries compared to similar months in 2019 (64 vs 33%), in part due to concerns about intrapartum transmission of SARS-CoV-2 (37.5%). With the COVID-19 pandemic outbreak, the delivery ward in our hospital was reorganized so it could safely accommodate pregnant women infected with SARS-CoV-2. The sudden increase in the infection rate in the pregnant population covered by our hospital, potentiated by limited human resources and uncertainty (at the time) about the impact of vaginal birth in vertical transmission, contributed to a substantial increase in the rate of caesarian deliveries. All pregnant women with COVID-19 and unfavorable Bishop score had an elective caesarian birth. Likewise, a high cesarean rate was described in other studies. (8), (9
Using the proposed Classification System for Maternal-Fetal-Neonatal SARS-CoV-2 Infections by Shah et al, we report two confirmed cases of congenital infection (4%), which is slightly higher than previously published data (3.2%).39 Consistent with other series, SARS-CoV-2 may be a cause of fetal death40 and further studies are needed to identify risk factors for vertical transmission.
In conclusion, our data shows that SARS-CoV-2 infection in pregnancy contributes to higher maternal morbidity due to higher cesarean rates and adverse perinatal outcomes in consequence of the higher rates of prematurity. Disease severity in pregnancy is mainly mild, with a low rate of maternal ICU admission. Lymphopenia may be an important marker for fetal distress and consequent iatrogenic preterm delivery, but further studies are needed to access this possibility.
Although data are reassuring in terms of maternal and neonatal mortality, vertical transmission does occur and may not be as innocuous as previously thought as it can lead to fetal death. Additional data from larger cohorts are needed to shed light into this subject.
Acknowledgements
We would like to thank all the physicians, nurses and other health care staff of the Obstetrics and Neonatal Department for the clinical management of the patients included in this paper.
Authors contributions
Conceptualization: Mariana Crespo Marques, Rute Branco, Margarida Meira de Carvalho. Methodology: Mariana Crespo Marques, Rute Branco, Margarida Meira de Carvalho. Formal analysis: Mariana Crespo Marques, Rute Branco, Margarida Meira de Carvalho. Data Curation: Mariana Crespo Marques, Rute Branco, Margarida Meira de Carvalho. Writing - Original Draft: Mariana Crespo Marques, Rute Branco, Margarida Meira de Carvalho. Writing - Review & Editing: Mariana Crespo Marques, Teresa Matos, Antónia Nazaré, Supervision: Teresa Matos, Antónia Nazaré.