Introduction
Gitelman’s Syndrome (GS) is a rare autosomal-recessive disease resulting from a mutation in the SCL12A3 gene on chromosome 16 (16q13), which translates into a loss of function of the encoded thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule of the kidney. It is the most common phenotype of hypokalaemic “salt-losing” renal tubulopathies. It is caracterized by typical acid-base and electrolyte disturbances like metabolic alkalosis, hypokalaemia, hypomagnesaemia and hypocalciuria. The increased sodium delivery to the cortical collecting duct leads to increased sodium reabsorption by the epithelial sodium channel, counterbalanced by potassium and magnesium loss resulting in hypokalaemia and hypomagnesaemia.1 A pregnant woman with GS has an increased risk of miscarriage, oligohydramnios and intrauterine growth restriction caused by often severe hypomagnesaemia and hypokalaemia. There is also a significant maternal morbidity due to difficulties in maintaining electrolyte balance. The approach and management become more challenging.
Case report
A 33-year-old G4P1A2 woman, caucasian, daughter of consanguineous parents, had medical history of asthma, overall muscular amyotrophy and menstrual disorders. No clinical data was found about the previous labor and abortions.
Since adolescence, she presents hypokalaemia with complaints of tiredness, asthenia, anorexia, ~ 10Kg weight loss, but without polydipsia, polyuria, diarrhoea, vomiting, diuretic or laxative therapy or eating disorders. She had no history of drug use other than transdermal contraceptive (norelgestromin and ethinyl estradiol). In May 2014, laboratory data revealed: serum K+ 3.4mmol/L, urinary K+ excretion >30mmol/day and elevated baseline serum renin. She was treated with oral potassium chloride, sometimes requiring intravenous therapy, and was referred to a Nephrologist.
The Nephrologist added up to 300mg bid spironolactone, 250mg bid magnesium aspartate and oral potassium supplements. In 2015, after several exams, the patient was diagnosed with Gitelman’s syndrome.
She became pregnant in March 2018, spironolactone was withdrawn and started 200mg/day eplerenone. Since then the patient went several times to the emergency room for nausea and vomiting caused by hyperemesis gravidarum, and at 27 weeks of pregnancy she was admitted to the gynaecology department with severe hypokalaemia (serum K+ 2.2mmol/L) and diagnosed with gestational diabetes, achieving glycaemic control by diet alone. Intravenous fluidotherapy, ondansetron and potassium chloride were administered. Four weeks later she was re-admitted for the same reasons. During pregnancy, electrolyte control was made twice or three times a month (Figure 1), and she had, at least, three ultrasounds that excluded fetal abnormalities. A normal spontaneous labor took place at 38 weeks and 3 days, without complications and a female child, weighing 2880-g was born in good condition. Our patient presented normal vital signs and there was no changes in electrocardiogram. During labor, potassium and magnesium control was made hourly by blood gas analysis and twice a day after birth.
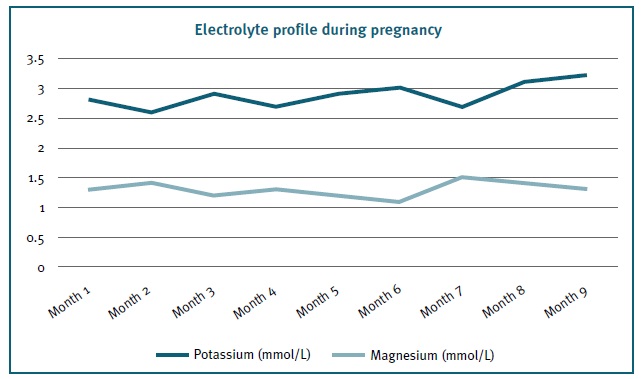
Figure 1 Potassium and magnesium seric values during pregnancy. Note that the lowest potassium value was 2.2mmol/L at 27 weeks (Month 7) coinciding with one of the hospital admissions. It should also be noted that the highest potassium values were obtained at the time of labor (3.5mmol/L).
During breast-feeding period, spironolactone was reintroduced at the same dose and blood analysis two months after labor showed: serum K+ 3.4mmol/L and Mg2+ 1.5mg/dL.
Currently, she remains symptom free and is medicated with 250mg bid magnesium and potassium aspartate supplements and 600mg bid potassium chloride.
Discussion and conclusion
SG is a rare autosomal-recessive condition and its prevalence is estimated at 1:40.000, resulting from the SCL12A3 gene mutation leading to a loss of function in NCCT (thiazide-sensitive NaCl cotransporter) in the distal convoluted tubule.2 Most patients are asymptomatic, although they may complain about muscle weakness, tetany, vertigo, thirst, nocturia, paresthesia and pain.3 Although it is a relatively benign condition, it may complicate with arrhythmia, seizures and persistent electrocardiographic anomalies. (4 The typical findings are metabolic alkalosis, hypokalaemia, hypomagnesaemia and hypocalciuria. Hyperreninaemia and secondary hyperaldosteronism may occur.5 The possibility of bilateral nephrocalcinosis is also described5 in addition to the pyrophosphate crystals deposition that can cause acute secondary arthropathy (pseudo-gout) as an initial or late manifestation. Treatment consists in potassium and magnesium replacement, and in more severe situations, drugs like aldosterone and amiloride antagonists.
During pregnancy there is activation of the renin-angiotensin-aldosterone system (RAAS), resulting in plasma volume expansion, increased renal blood flow and, consequently, increased glomerular filtration rate, and tubular function changes due to hormones. Fetal growth and high maternal levels of progesterone and estrogen induce a dynamic state with changes in electrolyte balance throughout pregnancy. Potassium excretion during pregnancy is low, possibly due to high levels of progesterone that may act as a protective mechanism.6 However, in GS this mechanism may be insufficient to compensate for ion loss.
GS in pregnancy has been associated with risk of spontaneous miscarriage, oligohydramnios and intra-uterine growth restriction7,8 as well as significant maternal morbidity due to difficulty in maintaining electrolyte balance.5 Women with chronic hypokalaemia are at risk of severe symptomatic electrolyte changes during pregnancy, especially if they have vomiting or diarrhoe.9 In addition, fetal needs may further exacerbate hypokalaemia. Although the diagnosis of GS is more common in adolescent or young adult, during pregnancy it is important to be alert to different disorders that mimic symptoms secondary to low potassium levels.
Treatment consists in electrolyte replacement at doses higher than usual. The use of aldosterone antagonists, spironolactone (category C drug) and eplerenone (category B drug), is not universal. Despite the known anti-androgenic effects of spironolactone, there are no reported cases of newborns malformations or fetal feminization.2 The risk appears to be lower with eplerenone, but evidence is rare.10 There are also cases of success reported with the use of eplerenone or amiloride (category B drug). RAAS blocking agents and prostaglandin synthetase inhibitors are not recommended for their high teratogenic risk. A case report showed that the patient never reached normal levels of potassium and magnesium during pregnancy, and always remain asymptomatic and without compromising fetal development.11 A single study defends that taking into account the salt-losing disease and, therefore the inability to maintain extracellular volume, the best treatment target would be fluidotherapy than traditional potassium and magnesium supplementation.1 In the most severe cases, the use of some drugs should be prioritized even if controversial, given the high risk of maternal death.12
The absence of laboratory data regarding our patient previous pregnancies does not allow us to conclude that this successful pregnancy resulted from the aggressive electrolytic approach and treatment. According to published cases, serum K+ ≥3mmol/L and magnesium within or near the lower range are recommended. Frequent blood analysis is fundamental. Normal serum potassium and magnesium levels are not necessary for a good obstetric result. Besides, achieving normal potassium levels is extremely difficult. In many cases, oral supplementation is not sufficient, forcing intravenous therapy.
Ideally, follow-up should start before baby conception to inform about the need for increased supplementation dose, risks associated with potassium sparing drugs and possible symptom exacerbation due to electrolyte alterations.13 A planned labor with appropriate cardiac monitoring will be prudent. This disease in pregnant women may have higher risks of fetal and maternal complications, although it has a good outcome if there is tight laboratory control. A case series of children currently with age between 6 months and 18 years-old born of women with GS showed that they are healthy and have normal neurological and somatic development.14
Electrolyte monitoring and a more aggressive oral and intravenous therapeutic approach are recommended in order to provide an appropriate environment for fetal development and maternal health. The multidisciplinary approach is essential.
This research did not receive any specific Grant from funding agencies in the public, comercial, or not-for-profit sectors.














