Case description
We present the case of a 28 years old female patient, nulliparous, diagnosed with FA at 14 years old. Genetic testing showed homozygous presence of variant c.2870G>A in the FANCA gene. Since then, she was followed at the Portuguese Institute of Oncology in Lisbon and had regular laboratory monitoring and performed bone marrow assessments annually. Over the years, laboratory monitoring showed non-progressive findings: hemoglobin ranging 11-13g/dl, average globular volume around 100fl (vitamin B12 and folic acid deficits excluded) and mild thrombocytopenia (platelets 110x103/uL). Sequential bone marrow tephrine biopsy, showed hypocellular marrow without myelodysplasia, leukemia or clonal changes. Other medical specialties performed regular evaluations with normal findings.
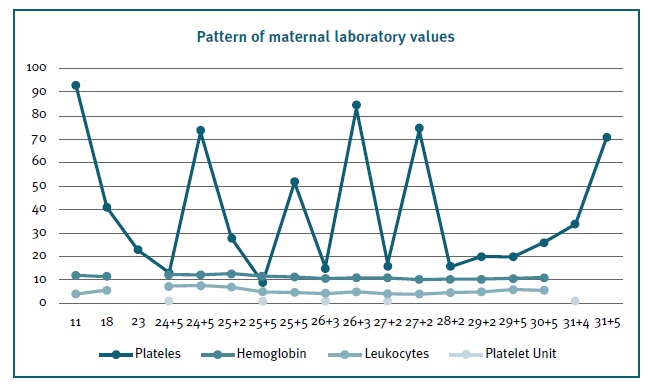
Pregnancy developed spontaneously. The first trimester was uneventful, and the blood tests showed mild thrombocytopenia (93x103/uL platelets). As pregnancy progressed platelet numbers worsened (Figure 1). At 18 weeks, with moderate thrombocytopenia (41x103/uL platelets), the patient was started on prednisolone 20mg/day.
Second trimester ultrasound didn’t reveal any malformations. At this time platelets declined further to 23x103/uL. From hereafter, patient’s blood tests were evaluated weekly. Prednisolone dose was further adjusted to 30mg/day, but without any improvement.
At 24 weeks, severe thrombocytopenia (13x103/uL platelets) developed, corticosteroid was discontinued, and platelet transfusions were needed. Despite a good response after the first transfusion (increment to 74x103/uL platelets), she soon became transfusion dependent, increasing their regularity to biweekly two weeks after.
Given the clinical worsening, the need to optimize the platelet count at delivery, and to avoid regular transfusions with alloimmunization risk, a multidisciplinary team decided, at 27 weeks, to begin Eltrombopag, a thrombopoietin receptor agonist (initially with 25mg, increasing a few days later to 50 mg/day, a dose that was kept). Subsequent weekly blood test evaluation showed that there was a good response to this therapy, maintaining platelet values around 20 x103/uL. No other transfusions were required until delivery.
In regular laboratory monitoring of the second trimester pregnancy, gestational diabetes was diagnosed, and a probable iatrogenic etiology by regular administration of hydrocortisone pre-transfusion can’t be excluded. It was subsequently controlled only with self-monitoring of blood glucose and diet plan.
At 29 weeks a fetal growth restriction (FGR) was diagnosed, and two weeks later the estimated fetal weight was in the 3rd percentile with Doppler changes. The patient was hospitalized and fetal pulmonary maturation begun. Two days later, delivery was planned due to new Doppler worsening. At that time, the patient had 34 x103/uL platelets and another platelet transfusion was performed that enabled a value of 71 x103/uL platelets before surgery. She ended up receiving 5 units of platelet concentrate during the pregnancy, with no record alloimmunization. At 31 weeks, a caesarean section was performed without complications, and a male infant with 1250g was delivered with an Apgar score of 5/8.
On the first day postpartum platelets were 77x103/uL. The puerperium was uneventful. The newborn was admitted to Neonatology due to very low birth weight and respiratory distress syndrome. Later he was discharged without complications or re-hospitalizations.
Three weeks after delivery, platelet numbers were back to baseline (120x103/uL). Eltrompobag was maintained up to 6 weeks postpartum. Platelets remained 100-150x103/uL after its suspension.
Discussion
FA is a rare inherited heterogeneous disease caused by mutations in one of at least 17 different genes, FANCA being the most common (60-65% of the cases). FA proteins’ major function is to maintain genomic stability, through the properly repair of DNA interstrand crosslinks6. The disease leads to bone marrow failure, increased sensitivity to cytotoxic therapies and malignancy predisposition2,5-8.
Patients usually present pancytopenia during the first decade of life but the diagnosis of the disease may not be achieved until adulthood because variable manifestations of the disease are common. Bone marrow failure eventually occurs in the majority of patients, though the time to onset can be quite variable, and in some cases only a single cell line will be involved (typically thrombocytopenia). This reflects our patient’s case who was diagnosed at 14 years old and presented only thrombocytopenia. Severe neutropenia and thrombocytopenia can lead to potentially life-threatening infections and bleeding, usually the cause of death of these patients2,5,9,10.
Congenital malformations occur in 60 to 75 percent of patients and are the most frequently presenting features of disease. The most common are skin findings (hyper- or hypopigmentation or café-au-lait spots), short stature and thumb or other radial abnormalities1,3,15. However, just like our patient, many patients with FA don’t manifest classical physical findings of the disease. In fact, about 40% of patients have no major physical anomalies7,15.
FA patients may have a range of endocrine disorders resulting from anatomical disruption of the hypothalamic-pituitary axis during development, more frequently: hypothyroidism, diabetes and dyslipidemia.Infertility and abnormal progression of puberty are frequent11,12. Premature ovarian failure occurs in over 75%. Hematopoietic stem cell transplant is the only treatment in the event of bone marrow failure1-3. Recently, there has been a reduction in the transplant associated toxicity, this allows a normal ovarian function recovery and a viable pregnancy4.
The heterogeneous manifestation of this disease helps to explain why our patient had a normal puberty and became spontaneously pregnant despite the high rate of infertility associated with this condition. The infertility, the low incidence of the disease, and low survival rate, make pregnancy a rare condition in these patients, making it difficult to establish an optimal course of follow-up.
During pregnancy, thrombocytopenia is the most common hemostatic change and carries a high risk of unfavorable outcomes: miscarriages, preeclampsia/eclampsia, FGR and fetal, neonatal or maternal death9,11. This is due to physiological mechanisms: increased volume of distribution; increased peripheral destruction by the placenta; and hormonal suppression of bone marrow.
In our patient’s case, her baseline thrombocytopenia due to FA, usually caused by a decrease in platelet production, was worsened due to the reported thrombocytopenic potential of the pregnancy itself. A multidisciplinary team decided to start a thrombopoietin receptor agonist, Eltrombopag, in face of a severe thrombocytopenia without corticosteroid response (which excluded a possible association with an autoimmune component), and the need for recurrent transfusions with alloimmunization risk. Eltrombopag is a thrombopoietin receptor agonist that leads to increased platelet production and is a successful treatment used in cases of refractary idiopathic thrombocytopenic purpura (ITP) that has been used before during pregnancy13.
The administration of this drug made it possible to maintain a stable platelet level with no need of recurrent platelets transfusion, reducing the risk of maternal fetal complications resulting from severe thrombocytopenia.
However, this is a difficult decision as, like most drugs, there are no adequate and well-controlled studies of its use in pregnancy (pregnancy category C by the Food and Drug Administration). Animal studies showed evidence of embryo lethality and reduced fetal weights at maternal toxic doses13,14.
Eltrombopag has oral administration and in
ITP should be initiated with a 50mg/day dose that can be increased to 75mg/day to maintain platelets ≥50×103/L14,15. In this patient, although these numbers were not achieved, the 50 mg/day dosage was kept considering the unknown risk of the drug in pregnancy and the early diagnosis of FGR. Since the drug was started during the third trimester, there was no risk of embryo lethality. Its administration allowed to reduce the need for recurrent transfusions and thus reduce the associated maternal and fetal complications.
Although FGR was a possible adverse effect of the Eltrombopag, we considered it more likely to be primarily due to the underlying maternal pathology, as it was detected early in relation to the drug administration.
Pregnancy in FA patients is a rare event due to reduced average life expectancy and decreased fertility. However, as our work shows, pregnancy is possible, even in non-transplanted women, with proper multidisciplinary medical follow-up essential to manage not only maternal and fetal risks of complications but also the necessary supportive therapy.
Although not yet described in the literature for FA, and further studies are needed, our work suggests that the use of a thrombopoietin receptor agonist in these cases of refractory cytopenia may, as in ITP, contribute to improved obstetric outcomes.