Background
Placenta Accreta Spectrum (PAS) is a relatively new umbrella term implemented in 2019 in the consensus guidelines developed by the International Federation of Gynecology and Obstetrics (FIGO), used for describing a complex disorder of placental development. There are three grades of PAS1, that depend on the depth and severity of myometrial invasion: in accreta, rather than being restricted within the decidua basalis, the chorionic villi attach to the myometrium; in increta, the chorionic villi invade the myometrium; in percreta, this invasion goes through the perimetrium, to and beyond the uterine serosa.
The most consensual explanation regarding its etiology hypothesizes that a defect of the endometrial - myometrial interface leads to an abnormal decidualization in an area of scar tissue, which allows abnormally deep placental anchoring villi and trophoblastic infiltration1,2.
Although still infrequent, PAS disorders are a growing concern and represent one of the most important causes of maternal morbimortality in modern obstetrics. They present a potentially life-threatening event, especially if not detected before delivery, as they affect the normal detachment of the placenta during the third stage of labor. Massive obstetric hemorrhage is one of its major complications, possibly leading to hemorrha-gic shock and coagulopathy, often needing a peripartum hysterectomy to prevent maternal death. They can also be a cause of injury to the surrounding organs1,2.
Understandably, it can additionally have a tremendous psychological impact, with increased risks of fertility loss, prolonged hospital admission, and overall morbidity3, which increase the risk of perinatal mental health disorders in a period of increased vulnerability to these conditions.
Prenatal diagnosis is key because it provides an opportunity to optimize management and outcomes. In PAS, maternal morbimortality is significantly reduced when the delivery is planned and occurs in a specialized center, under the care of a multidisciplinary team4. This approach is associated with shorter operative time, decreased maternal hemorrhagic morbidity, and fewer intensive care unit admissions, as well as better neonatal outcomes5,6.
The referral of suspected cases has been suggested as one of the most important measures in determining both maternal and neonatal outcomes7, thus implying that it is possible to optimize outcomes, even among women with a clinical high-risk profile. This referral, however, hinges on the identification of susceptible women and on precise prenatal assessment.
Still, recent population studies have shown that PAS disorders remain undiagnosed until delivery from half to two-thirds of the cases2, and are usually identified during labor when the placenta is retained, and/or there is heavy hemorrhage while attempting to remove it manually8.
Its contemporary global trend highlights the clinical need for an effective systematic screening guideline for this disorder in referring healthcare settings. Recently, studies have shown the combination of prenatal imaging with maternal and pregnancy risk factors improves the predictive accuracy of both the presence and severity of PAS9-13.
The major clinical factors that increase the risk of developing PAS are widely recognized, and most cases are associated with placenta previa (PP) and a history of cesarean section (CS) delivery. Other risk factors have also been identified, such as maternal age, multiparity, uterine interventions, and assisted reproductive techniques (ART) 1.
Ultimately, it seems evident that a more comprehensive understanding of the clinical profile of women at a higher risk of developing PAS disorders would represent a significant development in guiding targeted prenatal screening efforts and increasing the rate of antenatal diagnosis.
Objectives
To perform a systematic review and meta-analysis of the possible major clinical risk factors for PAS and estimate their risk. The primary goal is to establish the clinical profile of the woman at increased risk, combining the clinical risk factors associated with PAS into a practical screening guideline.
Methods
Study design
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and flowchart. The study protocol was registered in PROSPERO with the registration ID CRD42023360340.
The research question was defined using PICO principles: the population was the general obstetric population; the interventions/exposures were the following maternal clinical risk factors: number of previous cesarian deliveries, presence of placenta previa, maternal age, parity, maternal body mass index (BMI), previous uterine intervention, ART, hypertensive disorders and history of smoking during pregnancy; the comparator was the absence of the previously named risk factors; the main outcome was the occurrence of PAS.
We included both the cases where a histopathologic confirmation was obtained - if a hysterectomy was performed -, and the clinically diagnosed cases of PAS - categorized into “clinical PAS” -, when a conservative approach was taken, which included at least one of the following: (1) the absence of placenta separation 30 minutes after vaginal delivery, despite active management in third-stage labor, including intravenous infusion of synthetic oxytocin, uterine massage, and controlled cord traction, (2) difficult manual or fragmentary removal of the placenta and heavy bleeding from the placentation site, with partial or no placental separation during delivery and (3) evidence of gross placental invasion intraoperatively.
Search Strategy
A literature search was performed in three databases - PubMed, EMBASE, and The Cochrane Library -, between June 2022 and December 2022, using the Medical Subjects Heading Terms (MeSH) and with the following search phrases - for PubMed: ((placenta increta) OR (placenta percreta) OR (placenta accreta) AND (risk factors)); for Embase and Cochrane CENTRAL: ((placenta accreta spectrum) AND (clinical risk factors)).
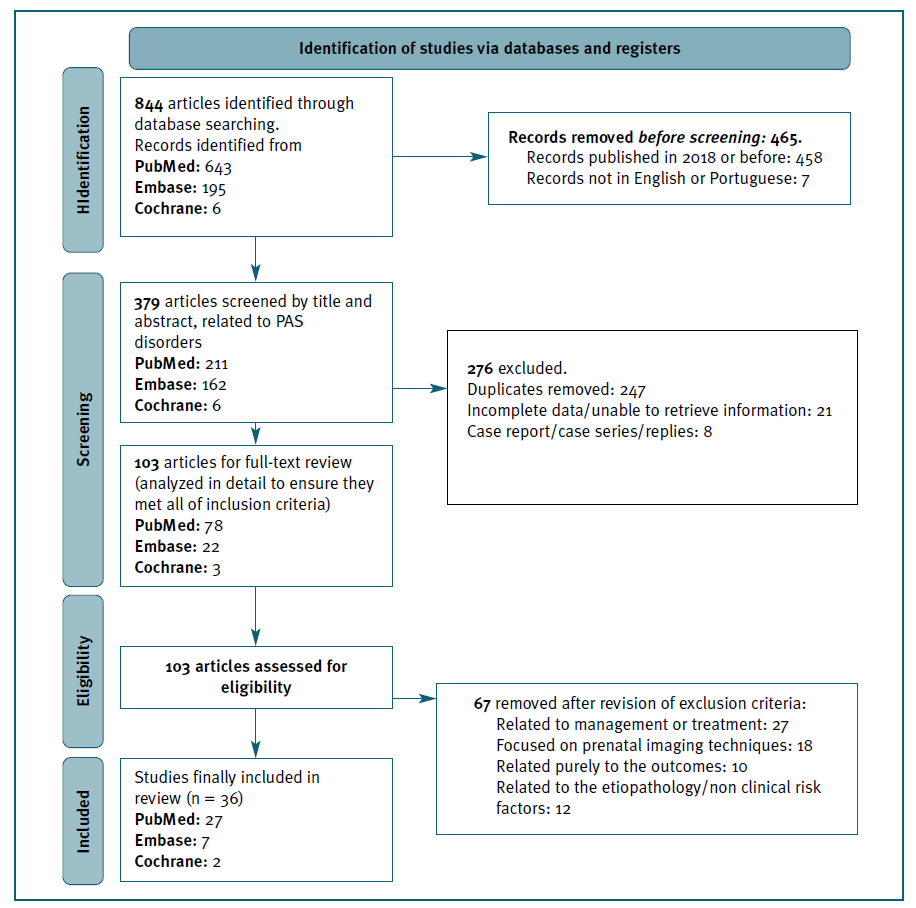
The PRISMA flow diagram for the identification of studies is presented in Figure 1.
Eligibility criteria
Studies that fulfilled the following inclusion criteria were included (1) articles focused on the clinical risk factors associated with PAS, confirmed clinically and/or histopathologically, in the general obstetric population, (2) studies that were published in 2019 and beyond, after FIGO’s Clinical Grading System was established, to support the process of clarifying reported data on PAS in international literature, (3) studies limited to the English and Portuguese languages (4) studies limited to human subjects.
Data Extraction and Study Selection
The remaining articles were then independently screened for inclusion criteria by two reviewers (A.S and I.S.S), based on titles and abstracts. The reasons for excluding trials were recorded. Next, a screening of the full-text reports of the remaining articles was performed, after which it was decided whether those met the inclusion criteria.
The eligible articles were analyzed and information was extracted: study characteristics (first author, publication year, study design, country, sample size), participant characteristics (population demographics and risk factors), measure used for the point estimates (OR). Data were stored on Microsoft Excel spread-sheets.
With the extracted data, a meta-analysis was performed when possible. A random effects model was used, and the results were presented in forest plots. To assess heterogeneity between studies, Cochran’s Q test and the I² index were used. The analysis was performed in R using the package metafor, and a significance level of 0.05 was considered. Forest plots were produced in MS® Excel® from the R results.
Quality Assessment for the included studies was performed following adapted versions of assessment Scales by the American National Heart, Lung and Blood Institute (NHLBI).
Results
A total of 36 studies were finally included in the systematic review, the majority of which were conducted in China and the USA, followed by Italy. In “maternal age”, “number of fetuses/multiple gestations” and “hypertension disorders”, we did not find sufficient data to perform a meta-analysis.
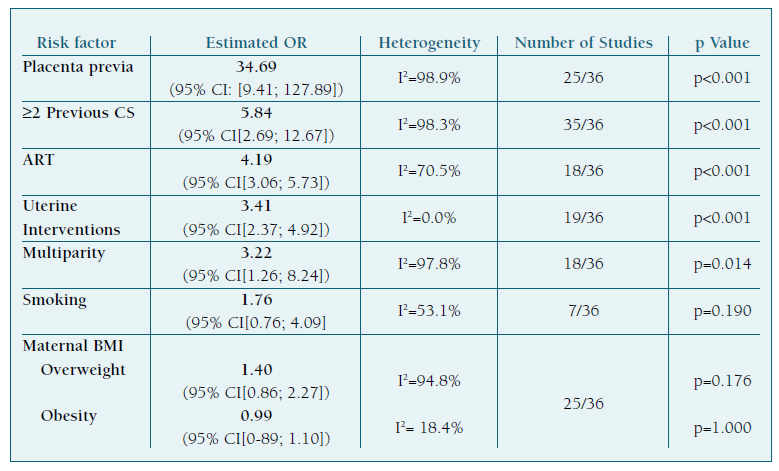
We found that PP (OR 34.69, 95% CI[9.41; 127.89]), a history of two or more previous CS (OR 5.84, 95%CI[2.69; 12.67]), ART (OR 4.19, 95%CI[3.06; 5.73]), uterine interventions (OR 3.41, 95%CI[2.37; 4.92]), and multiparity (OR 3.22, 95%CI[1.26; 8.24]) were risk factors for the development of PAS disorders. Results regarding maternal BMI and smoking during pregnancy were not statistically significant.
A table of summarized results is presented in Table I.
Discussion
Women with the combination of placenta previa and at least one prior CS have clearly and consistently been identified as a high-risk clinical profile for PAS1,14,15. Our results support these findings, as these two risk factors had the biggest estimated OR. The work of Conturie and Lyell16 also found that women with both placenta previa and a history of cesarean delivery are more likely to have a higher grade of invasive PAS. Both conditions are identified as independent risk factors for PAS and their identification plays a critical role in both its diagnosis and optimal management.
Placenta previa emerged as the most significant finding in our study. Although it cannot be prevented, its early detection and meticulous management are crucial for achieving the best possible outcomes. These patients require close monitoring throughout their pregnancy and may need a planned cesarean delivery to prevent complications.
Our results also found that a history of previous CS was associated with a higher risk of developing PAS. While we did not study this, other studies have also consistently identified a cumulative association between a previous CS and PAS17, highlighting its importance and significance as a major clinical risk factor. This is a particularly important finding because a history of previous cesarean delivery is the potentially modifiable risk factor most strongly associated with these disorders.
It is important to acknowledge that other factors can also contribute to the development of PAS - it is imperative that healthcare providers remain highly vigilant and consider PAS in women presenting with other known associated risk factors.
A recent meta-analysis showed that pregnancies after ART are associated with higher risks of both obstetric and perinatal complications when compared with spontaneous conception, including a significantly increased risk of abnormal placentation, especially for PAS and vasa previa19. Our results paralleled this and other recent studies, supporting the hypothesis that ART increases the risk of PAS.
Our results also supported previous studies that found that uterine interventions increased the risk of PAS15, as any procedure damaging to the integrity of the uterine lining can theoretically cause abnormal placentation20.
Our results also suggest that multiparity is a significant risk factor for PAS. Multiple pregnancies can cause changes in the uterine environment, such as uterine scarring or damage to the endometrial lining. Additionally, with each pregnancy, the uterine wall becomes thinner, making it easier for the placenta to invade and grow deeply into the uterus20.
When it comes to smoking during pregnancy, our results were not statistically significant. Nonetheless, other studies have found that smoking during pregnancy may increase the risk of PAS12,21-23 because it (1) impairs wound healing of scarred uterus and has been linked to changes in the structure and function of the placenta, which may increase the risk of abnormal placentation and (2) also increases the risk of placenta previa21.
It is known that women with advanced maternal age are at a higher risk of various adverse maternal and neonatal outcomes. The work of Humaira Ali et al. showed that the odds of PAS increased for every one-year increase in age in women with a previous cesarean24. Although these findings may be confounded by higher parity, placenta previa risk, and higher probability of a previous uterine intervention or fertility treatments, it may also represent an altered hormonal and/or implantation environment, as suggested by various studies where advanced maternal age was found to be an independent risk factor for PAS8,25,26. As delayed childbearing becomes increasingly more common, we argue that addressing maternal age and its possible association with PAS is important.
The occurrence of PAS has been recently reported to be higher in multiple gestations, possibly because there is excessive myometrium stretching and enlargement of the placenta9,27, although current data on this topic is still scarce.
The data on the association of hypertensive disorders and PAS is mixed and scarce. Because the presence of PAS has been linked to increase the risk of adverse outcomes in women with hypertensive disorders28, including severe bleeding and emergency hysterectomy, we recommend careful monitoring.
Regarding maternal BMI, it remains unclear whe-ther its association with PAS is independent of confounding factors, such as the higher risk of cesarean delivery commonly observed in obese women. As high maternal BMI can increase the risk of other various complications for both the mother and the baby, including labor complications that can make the management of a concomitant PAS disorder even more challenging, we suggest close monitoring of these women.
It is important to note that not all cases of adherent placenta require major surgery, and conservative management can be successful in some cases29. In patients with adherent placentas requiring manual extraction, the pathologic finding of focal accreta is associated with an increased risk of hemorrhagic morbidity and retained placenta in subsequent pregnancies30,31.
Our guideline
We believe that the key measure in optimizing both prenatal diagnosis and outcomes of women at increased risk for PAS is the referral of suspected cases, which implies that healthcare providers should know whom to refer and when to do it - and, thus, profiling women by their clinical risk is crucial.
We aimed to combine our findings and current knowledge on the clinical risk factors associated with PAS into a prediction guideline, to provide recommendations on the best approaches to the screening of PAS.
Our guideline defines who should be screened, who is responsible for performing the screening, the tool to be used and what defines a positive screen test, as well as what should be done with a positive result.
We suggest that the standardized routine prenatal care visits and ultrasounds present an unmissable opportunity to also actively screen for PAS risk factors. Additionally, preventive measures can be taken (and will ideally start during preconception counselling), such as: promoting vaginal delivery, which includes encouraging women with a previous CS to attempt trial of labor for subsequent births, if clinically safe; counselling about nutrition and exercise and support smoking cessation; advising women considering undergoing ART; and addressing the role of maternal age on reproductive planning and outcomes.
All pregnant patients should be screened, and the screening process can be carried out by any healthcare professional, who should continue to search for risk factors and any alterations during every prenatal care visit and routine ultrasound. We propose starting by implementing the checklist suggested in Table II and acting according to its final score.
A score lower than 2 points defines a low-risk clinical profile. We suggest maintaining a screening program similar to any other pregnant woman.
Table II clinical risk factors to consider and respective final clinical risk profile for PAS disorders.
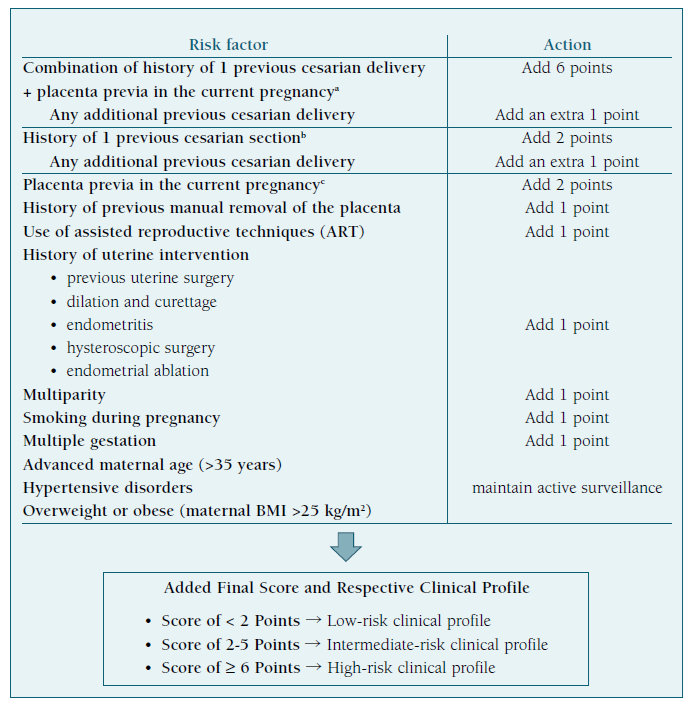
A score between 2 and 5 points defines an intermediate-risk clinical profile - if placenta previa is suspected or identified, we suggest women should undergo regular ultrasound examinations during the second and third trimesters to confirm persistent previa and consider referral.
A score of 6 points or more defines a high-risk clinical profile and we recommend immediate referral to a specialized diagnostic center, experienced in PAS disorders.
The main strengths of our work are that this triage of women: (1) can be carried out by any healthcare provider, in both a primary care setting and a medical facility with limited resources, (2) does not necessarily require additional medical appointments beyond those that are already scheduled for prenatal general care, (3) proposes a score that considers the different relative clinical importance of each risk factor, attributing points according to its clinical significance and (4) as a screening test, it can identify potential health problems before they become life-threatening conditions, which in turn can reduce associated healthcare costs, thus improving preventive care and overall health equity by increasing early access to affordable healthcare services.
The main limitations are: (1) our guideline will need validation and (2) our meta-analysis was complicated by heterogeneous subsets of women and methodology across studies. High heterogeneity can make it challenging to draw definitive conclusions and may require additional methods such as subgroup analysis or meta-regression to try and identify and explore potential reasons for the variation in effect sizes between studies. Furthermore, since the sum of patients was small for some of the studied risk factors found in these studies, conclusions should be interpreted with caution.
Nevertheless, the guideline’s validation may be performed using simulation tests and, thus, it has the potential to become a great clinical instrument in the future. We also argue that the high heterogeneity in current literature emphasizes the need for a standardized and consensual method for diagnosing and reporting PAS.
Conclusion
Safe and effective care of a woman with a PAS disorder depends on a timely diagnosis. An efficient and organized screening program for PAS is crucial in referring medical settings, as it would enable early detection and referral of high-risk women to PAS diagnostic centers. A high level of suspicion is necessary for early diagnosis and, in this, profiling the women with known relevant risk factors is essential.
Our results directly impact the ability of screening - with the proposed guideline, we can identify the women that will benefit from an early referral to experts, which may increase the rate of antenatal diagnosis and reduce PAS-related complications with proper planning and follow-up.
Authors’ contributions
Anaísa S. Simões: conceptualization, data curation, methodology, writing - original draft, review and editing; Isabel Santos Silva: conceptualization, data curation, supervision, writing - review and editing; Francisco Caramelo - formal statistical analysis and validation, supervision, writing - review and editing.