Introduction
Infertility affects more than 48 million couples worldwide, with important medical, psychological and socioeconomical consequences1. Assisted reproduction technologies, such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), are amongst the possible treatments used in these situations. In most centers, these techniques require controlled ovarian stimulation to achieve a multifollicular development2. The fast-rising estradiol levels associated with the simultaneous development of multiple oocytes may cause a premature luteinizing hormone (LH) surge, compelling the administration of gonadotropin-releasing hormone (GnRH) analogues3.
Over the past decade, ovarian stimulation with gonadotrophins and LH surge suppression with GnRH antagonist has become the most widely protocol used in IVF/ICSI cycles, due to its shorter duration, lower number of injections and greater clinical safety compared to GnRH agonist protocols, improving patient compliance4,5 without compromising treatment outcomes3,6-8. The main disadvantage lies in the lack of flexibility on the starting day of gonadotrophin stimulation as it is dependent on spontaneously occurring menses if a fresh embryo transfer is anticipated4,9,10. This inability to adequately program the cycle dates may lead to a higher frequency of weekend oocyte retrievals, which is less than ideal for both the patients and the staff.
The increased duration of the workweek can lead to physical and psychological stress, burnout and work demotivation, which may have an adverse impact on the quality of the work performed. Nonetheless, several studies failed to show a correlation between working during the weekend and suboptimal results11,12. Even though treatment outcomes do not appear to be compromised, it jeopardizes the distribution of the workload throughout the week and the management of human resources, reducing the centers’ efficiency and increasing its costs.
Previous experience with IVF/ICSI cycles using GnRH agonist protocols has demonstrated that scheduling the starting day of ovarian stimulation is a useful strategy to avoid weekend oocyte retrievals11,13-16. There are several types of pre-treatment therapies in IVF protocols that allow changing the first stimulation day: estrogen or progesterone supplementation, combined hormonal contraceptive (CHC) methods (oral, transdermal or vaginal), GnRH antagonist administration with or without estrogen priming, and GnRH agonists. In some assisted reproduction centers, hormone pre-treatment before GnRH antagonist regimens is being used as a strategy to program the beginning of the cycle9,13,17-24. However, its impact on pregnancy and live birth rates remains controversial. The safety of pre-treatment modalities was recently issued in the European Society of Human Reproduction and Embryology (ESHRE) guidelines on ovarian stimulation for IVF/ICSI (The ESHRE Guideline Group on Ovarian Stimulation, 2020) 25. While a Cochrane meta-analysis26 reported a negative effect on these rates, other studies27 found no significant differences. Additionally, some researchers28 suggest that extending the washout interval may help mitigate any potential negative effect on endometrial receptivity or oocyte quality. Despite these controversies, hormone pretreatment continues to be widely adopted in clinical practice due to its practical benefits in cycle scheduling. A 3-day pretreatment course with a GnRH antagonist in the early follicular phase in a GnRH antagonist stimulation protocol is also used in several centres and the number of oocytes retrieved and reproductive outcomes did not vary significantly23,29. Moreover, although most of these studies start gonadotrophin stimulation on a Friday9,13,18,21, scientific evidence supporting this practice is lacking and the best day to start the cycle to avoid weekend retrievals has not been conclusively determined.
Therefore, the aim of this study was to determine the best weekday to start gonadotrophin ovarian stimulation to reduce the frequency of weekend oocyte retrievals in IVF/ICSI cycles using GnRH antagonist protocol when a fresh embryo transfer is anticipated, as well as to evaluate the impact of the day of oocyte retrieval in laboratory outcomes.
Methods
This study followed the reporting guidelines from the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement for cohort studies30.
Study design, Setting and Participants
A retrospective cohort study of IVF/ICSI cycles with GnRH antagonist protocol was performed in the Reproductive Medicine Unit of a Portuguese University Hospital.
All consecutive women submitted to oocyte retrieval between January 2016 and December 2021 after ovarian stimulation with gonadotrophins and LH surge suppression with GnRH antagonist protocol and a fresh embryo transfer intent in the beginning of the stimulation were included. All women with cancelled cycles due to inadequate response to gonadotrophin stimulation or errors in medication administration, as well as IVF/ICSI cycles with GnRH agonist protocol and those receiving hormone pre-treatment with estrogen, progestogens or oral contraceptives for cycle scheduling were excluded. In the public healthcare system in Portugal, according to current regulations, IVF/ICSI cycles are limited to women under 40 years of age and to a maximum of 3 cycles per couple. Therefore, all women included in the study fulfilled these conditions.
Ethical approval for this study was granted by the local ethics committee board (registration number 25/23). All patients gave prospective consent for their records to be accessed for investigational purposes such as this study. The data was collected retrospectively by reviewing the electronic and physical medical records and de-identified data was recorded and stored in a standardized computer spreadsheet.
IVF/ICSI clinical protocol
Gonadotrophin stimulation was initiated in the early follicular phase (2nd or 3rd day of the menstrual cycle) and the initial dose was individualized according to the patient’s anti-müllerian hormone (AMH) level, antral follicle count (AFC) and previous response to stimulation. Per protocol, a GnRH antagonist was administered from the 6th day of stimulation onwards to prevent premature LH surges. Follicular development was monitored by serial ultrasound examinations and, when at least 3 follicles reached 17 mm, final oocyte maturation was triggered with urinary human chorionic gonadotropin (hCG) (Pregnyl®), choriogonadotropin alfa (Ovitrelle®) or triptorelin (Decapeptyl®) depending on the risk of ovarian hyperstimulation syndrome. Transvaginal ultrasound-guided oocyte retrieval was performed 34-36 hours later.
The oocytes collected were inseminated or injected with highly purified sperm and, sixteen hours later, light microscopy examination was used to verify fertilization. A measurement of endometrial thickness was performed on the day of oocyte retrieval and 1-2 embryos were transferred transcervically between the 2nd and 5th day of embryonic development. Any surplus embryos were frozen if the blastocyst stage was achieved. A freeze-all strategy with deferred embryo transfer was used in patients with high risk of ovarian hyperstimulation syndrome or unfavorable endometrial features.
Variables and Data sources/Measurements
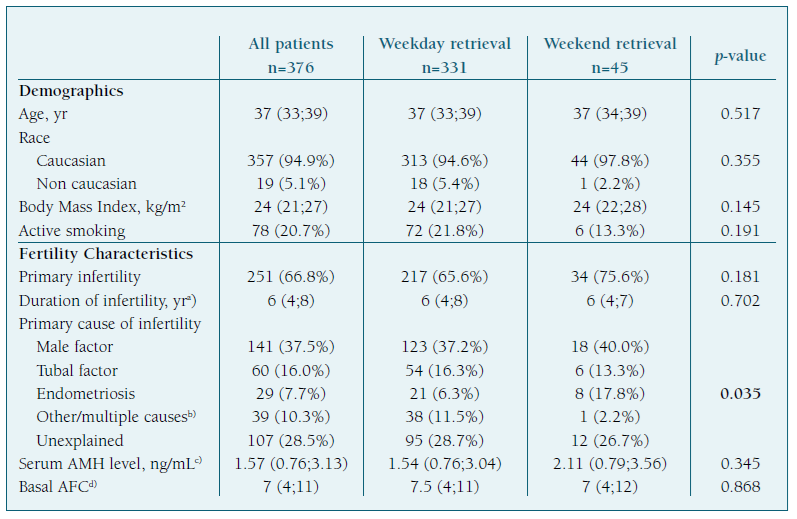
For each patient, data regarding demographics, clinical and cycle characteristics and laboratory outcomes were collected. Demographic and clinical variables included the patients’ age, race, body mass index, smoking habits, type, cause and duration of infertility, AMH level, AFC, weekday in which ovarian stimulation was initiated, duration and total dose of gonadotrophin administration, endometrial thickness, weekday of oocyte retrieval and assisted reproduction techniques used. The number of oocytes retrieved per cycle, fertilization rate (defined as the number of two pronuclei obtained per inseminated/injected mature oocyte on day 1) and number of viable day 2 embryos obtained were also evaluated.
Study outcomes
The main outcome was to determine which weekday for initiating ovarian stimulation resulted in the lowest number of weekend oocyte retrievals. Secondary outcomes included evaluating whether the number of oocytes collected, fertilization rate and number of viable day 2 embryos obtained were affected by the day of oocyte retrieval. For this sub-analysis, the cycles were divided into two groups: those in which oocyte retrieval occurred on a weekday and those in which oocyte retrieval was performed during the weekend.
Statistical analysis
Statistical analysis was carried out using STATA® version 16 (Statistics/Data analysis, StataCorp LLC, Texas, USA). All variables were addressed for normal distribution using Shapiro-Wilk test. Continuous variables were presented as median and interquartile range (IQR) when non-normally distributed and mean and standard deviation when normally distributed, and categorical variables in absolute numbers (n) and percentages. Comparisons between two groups were made using Chi-square or Fisher’s exact test for categorical variables and Mann-Whitney or t-test for continuous variables. Kruskall-Wallis test was used when more than two groups were compared. Results were considered statistically significant if a two-tailed p-value <0.05 was observed.
Results
Participants and descriptive analysis
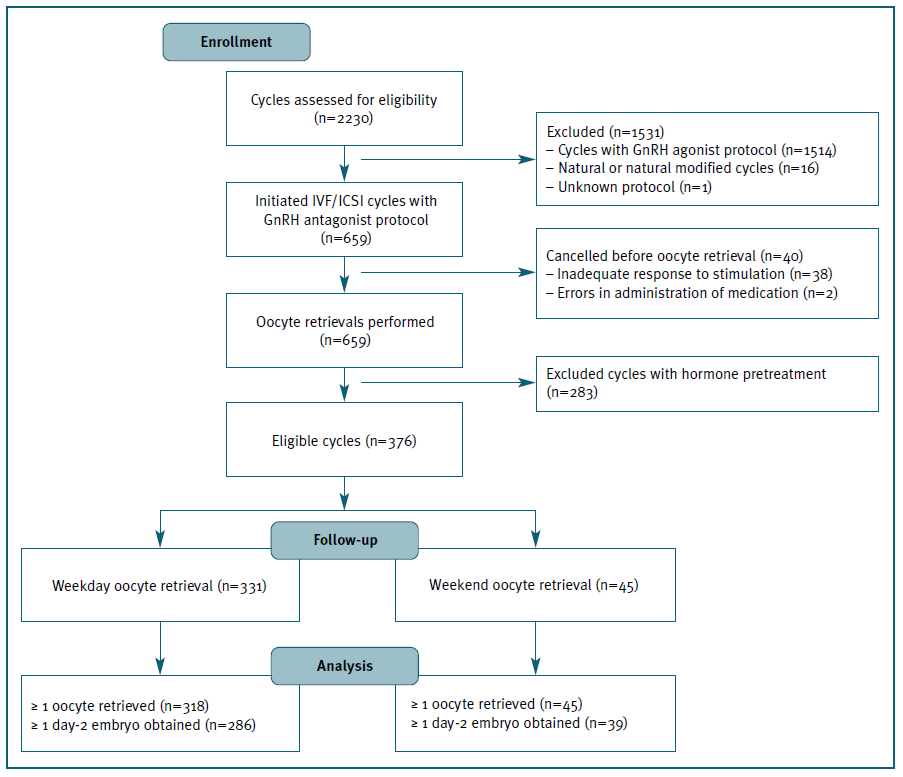
In the study period, 2230 IVF/ICSI cycles were initiated, 699 of which using GnRH antagonist protocol. Forty cycles were cancelled before oocyte retrieval and, out of the 659 follicular punctures performed, 376 met the inclusion criteria and were included in the analysis. (Figure 1)
Most oocyte retrievals were performed during the 5-day workweek (n=331; 88%), with the highest frequencies registered on Friday (n=97; 25.8%), Wednesday (n=75; 19.9%) and Monday (n=72; 19.1%), and only 45 (12%) occurred during the weekend. (Figure 2) Patients’ demographics and cycle characteristics are detailed in Tables I and II and were similar between women with or without weekend retrievals, except for the primary cause of infertility (p=0.035). (Tables I and II)
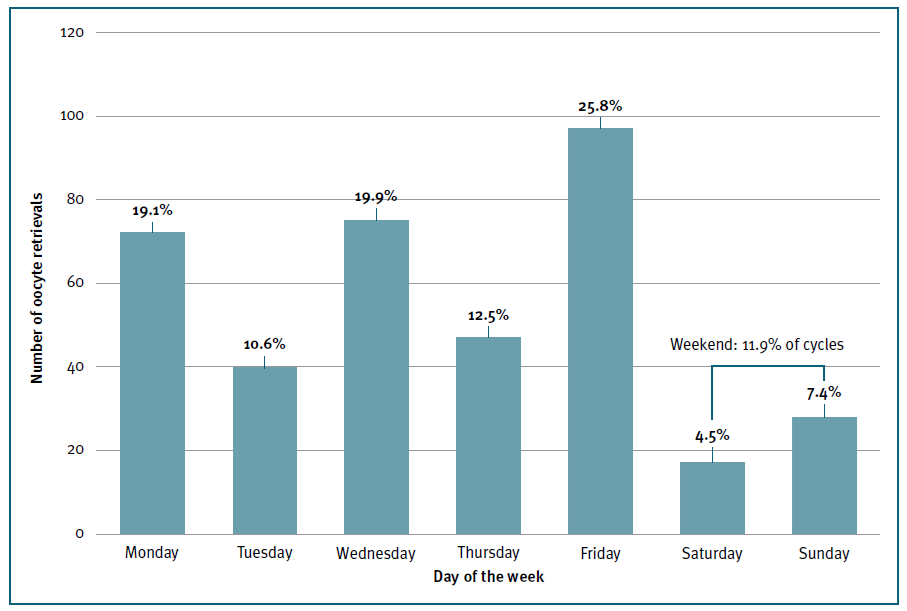
Figure 2 Frequency distribution of oocyte retrieval by day of the week. Y axis represents the absolute number of oocyte retrievals. X axis represents each day of the week. Relative percentages of the overall total number of oocyte retrievals are represented on the top of each column.
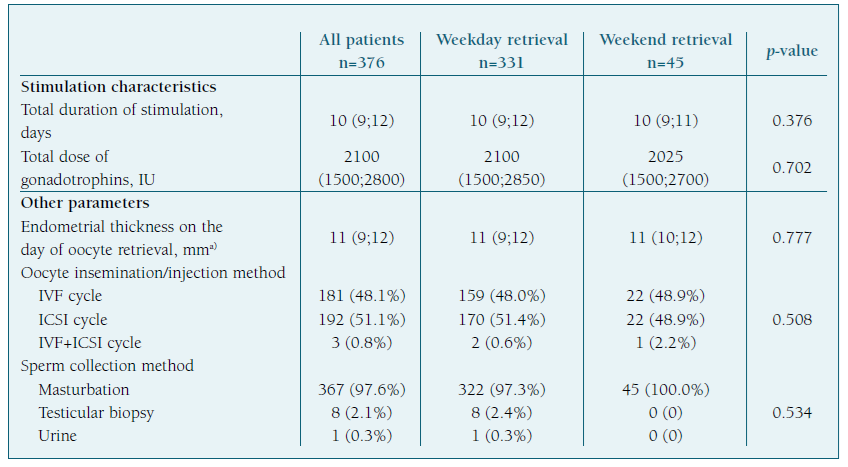
The first day of gonadotrophin stimulation occurred randomly across the days of the week, with a slightly higher percentage observed on Friday (n=90; 23.9%). (Figure 3) The median total dose of gonadotrophins administered per cycle was 2100 IU (IQR 1500;2800) and the median duration of stimulation required to achieve final oocyte maturation criteria was 10 days (IQR 9;12), with no significant difference after accounting for the day of the week of oocyte retrieval (p=0.23). (Table II)
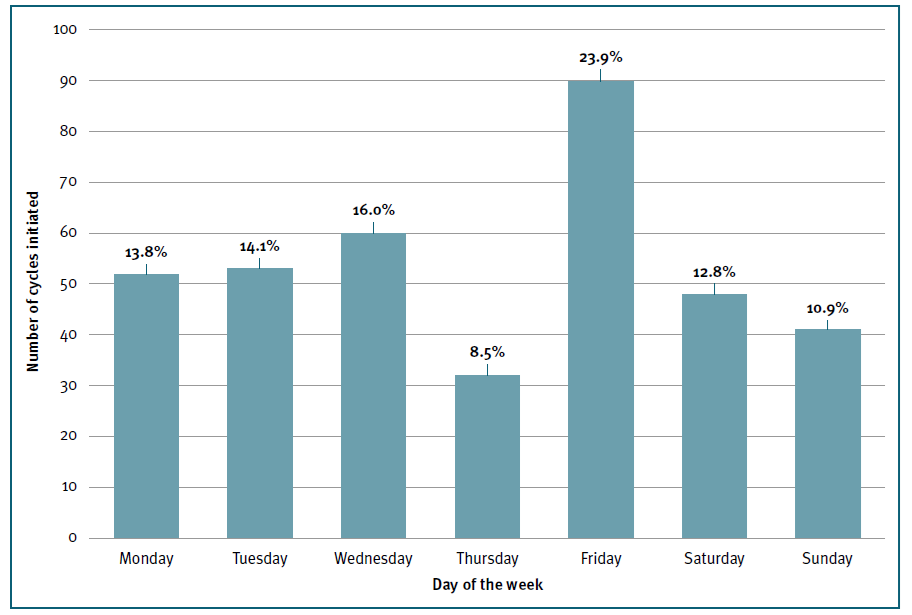
Figure 3 Frequency distribution of the starting day of gonadotrophin stimulation by day of the week. Y axis represents the absolute number of IVF/ICSI cycles in which gonadotrophin stimulation was initiated. X axis represents each day of the week. Relative percentages of the overall total number of cycles are represented on the top of each column.
Primary Outcome
The frequency distribution of the days of the week of oocyte retrieval relative to the first day of ovarian stimulation was analyzed to determine the best day to start the cycle to avoid weekend oocyte retrievals. Saturday and Sunday oocyte retrievals were less likely to occur if gonadotrophin stimulation started on Friday compared to the other days of the week (4.4% vs 14.3%; p=0.01) and more likely if started on Monday (p=0.002) or Tuesday (p=0.008). (Figure 4) No difference was found when stimulation was initiated on the other days of the week.
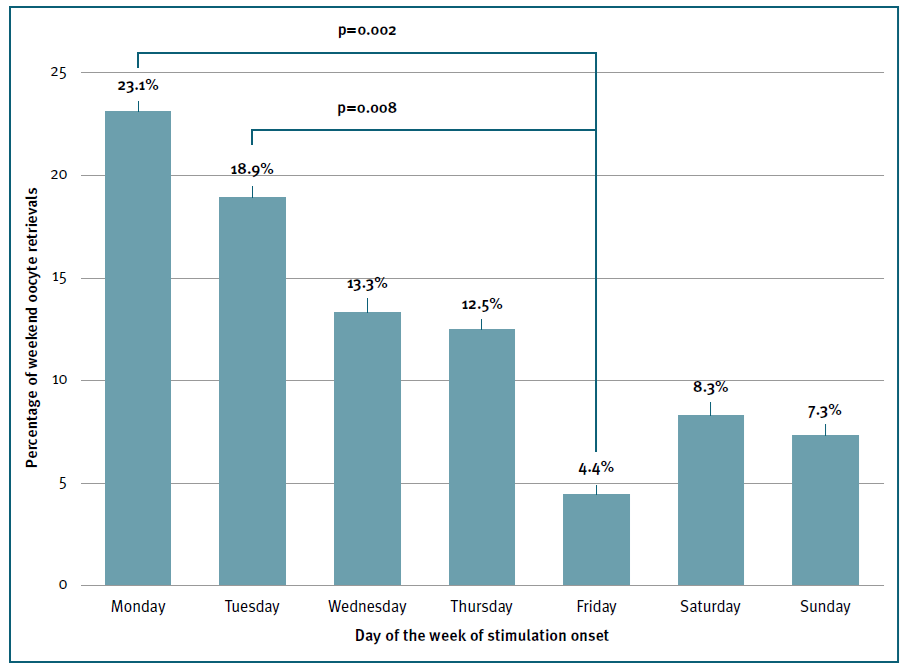
Figure 4 Frequency distribution and probability of weekend oocyte retrieval by day of the week of gonadotrophin stimulation onset. Y axis represents the percentage of weekend oocyte retrievals. X axis represents the day of the week in which gonadotrophin stimulation was initiated. Compared groups and p values are shown on the top of the columns.
Secondary Outcomes
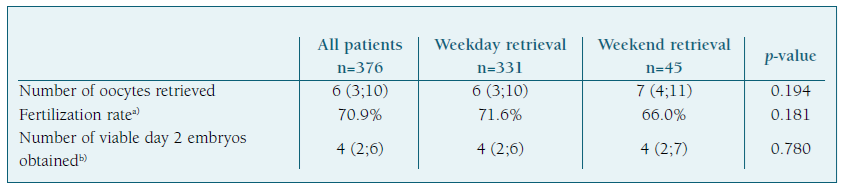
Treatment outcomes are presented in Table III and were analyzed separately according to the day of oocyte retrieval (weekday vs. weekend). Of the 376 ovarian retrievals studied, a median of 6 oocytes (IQR 3;10) were collected per procedure, with an overall fertilization rate of 70.9%.
The number of oocytes retrieved (p=0.194), fertilization rate (p=0.181) and the number of viable day 2 embryos obtained (p=0.780) were not influenced by the day in which oocyte retrieval was performed. Although the fertilization rate (66% vs. 71.6%) was lower in the weekend group, the difference was not statistically significant (p>0.05).
Discussion
The results of this study suggest that initiating ovarian stimulation on Monday and Tuesday should be avoided to reduce weekend retrievals and show that laboratory outcomes are not influenced by the day of oocyte retrieval.
In this center, as in many others, GnRH antagonist protocols gradually replaced the long down-regulation GnRH agonist cycles as the first-line therapy3,6, creating an increased pressure on the staff due to the inability to adequately program the cycle dates9. Several ideas emerged to improve cycle scheduling, such as programming the starting day of ovarian stimulation13,17-22,31,32. However, for this to be effective, knowledge of the average cycle length and the estimate of the best day to start gonadotrophin administration are crucial.
In our cohort, the median duration of gonadotro-phin stimulation was ten days, similarly to what is reported in other studies18,28,32-35. We also found a significant variability in the duration of ovarian stimulation among the included cycles (5-22 days; IQR 9;12), which may pose challenges to scheduling the cycle’s onset as a strategy to minimize working on the weekend.
To calculate the best day to start the cycle, we explored interaction effects between the first day of gonadotrophin stimulation and the day of oocyte retrieval. In our study, the onset of ovarian stimulation on Monday and Tuesday resulted in significantly more oocyte retrievals on the weekend compared to the cycles in which stimulation was initiated on Friday. These results were not explained by differences in the cycle length as they were similar after accounting for the duration and dose of gonadotrophin administration. Considering that ovarian stimulation can be initiated on the second or third day of the menstrual cycle when a fresh embryo transfer is planned, our results suggest that gonadotrophin administration should be started on the second day if the menstruation starts on a Saturday and on the third day if it begins on a Monday, with no difference in the remaining days of the week.
Furthermore, in addition to determining the optimal day to initiate gonadotrophin ovarian stimulation to avoid weekend oocyte retrievals, it is crucial to address the scheduling of fresh embryo transfers. According to the current guidelines36, day-5 embryo transfers should be prioritized. This means that oocyte retrievals in cycles planning for fresh transfers should ideally occur on a Wednesday, Thursday or Friday, ensuring that transfers can take place on a weekday the following week. By combining this information with the results of our study, we conclude that gonadotrophin stimulation should ideally be initiated on Friday, Saturday or Sunday. This strategy not only helps prevent weekend oocyte retrievals but also avoids fresh embryo transfers occurring on weekends, thereby enhancing the overall efficiency of Reproductive Medicine Units.
Twelve percent of the included oocyte retrievals were performed during the weekend, a significantly higher percentage than the frequency described in some studies where oral contraceptives were used for cycle scheduling17,20,21. In contrast to previous published studies37, a recent meta-analysis showed that the duration of gonadotrophin stimulation in cycles with or without hormone pre-treatment was similar27. As discussed earlier, the impact of hormonal pre-treatment before IVF/ICSI cycles using GnRH antagonist protocols remains controversial, with some studies showing a negative effect on pregnancy and live birth rates26, while others27 found no significant differences. An alternative strategy could involve the pre-treatment early administration of a GnRH antagonist29 starting on day 1 or 2 of the menstrual cycle, allowing for a delay in the initiation of gonadotrophin stimulation to a more optimal date. However, further analysis using this population is necessary to validate this hypothesis since all cycles involving hormone pre-treatment were excluded from our study.
Regarding treatment outcomes, as previously reported in the literature11,12, no difference was found between cycles with or without weekend oocyte retrievals.
Our study has a few limitations. Firstly, the retrospective single-center study design can be associated with various sources of bias. Secondly, the ovarian stimulation protocol used varied among individuals, with different types, doses and combinations of gonadotrophins administered, resulting in clinical heterogeneity. Thirdly, we did not evaluate whether oocyte maturation was triggered on an ideal day according to ultrasonographic follicle size criteria or if hCG administration was advanced or delayed avoiding weekend retrievals, thus a selection bias is possible. Additionally, there were statistically significant differences in the primary cause of infertility between the groups, which may influence the outcomes and should be considered when interpreting the findings. Lastly, we did not perform a subgroup analysis based on the different ovarian responses to stimulation and, given that most women in our study had a normal ovarian reserve, an adequate response to stimulation could be expected. This is likely to be relevant as it may influence the median duration of gonadotrophin stimulation and thus the most favorable day to start the cycle. It remains unknown how effective the application of our results would be in scheduling the cycle in a different population in which a faster or longer ovarian response is more likely to occur.
Overall, our findings suggest that to minimize weekend oocyte retrievals, ovarian stimulation should ideally be initiated on Friday, while avoiding Monday and Tuesday. For Reproductive Medicine Units aiming to further reduce weekend workloads, we recommend starting stimulation on Friday, Saturday or Sunday, as it maximizes the probability that both oocyte retrieval and day-5 fresh embryo transfers occur during the 5-day workweek. This might be achieved by choosing the first day of gonadotrophin administration according to the day of the week of the beginning of the menstruation or by using hormone pre-treatment to strategically program the beginning of the cycle. Our results also show that treatment outcomes are not influenced by the day of oocyte retrieval. Nevertheless, we still believe that cycle scheduling is an important matter to address to avoid 7-day working weeks, to optimize human resources and to avoid team burnout. These adjustments not only streamline clinic operations but also contribute to a more efficient and sustainable reproductive medicine environment.
As research on this area continues, more data will be available about the efficacy and safety of this practice and could lead to changes in current knowledge and recommendations.
Authors’ contributions statements
Margarida Meira de Carvalho: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Catarina Policiano: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Isabel Pereira: Investigation, Writing - review & editing. Carlos Calhaz-Jorge: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Giedre Lopes: Investigation, Writing - review & editing. Marta Carvalho: Investigation, Writing - review & editing. Fernanda Leal: Investigation, Writing - review & editing. Ana Aguiar: Conceptualization, Investigation, Methodology, Supervision, Writing - review & editing.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.