Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Angiologia e Cirurgia Vascular
versão impressa ISSN 1646-706X
Angiol Cir Vasc vol.13 no.3 Lisboa dez. 2017
ARTIGO DE REVISÃO
Inflammatory abdominal aortic aneurysm: review
Aneurisma inflamatório da aorta abdominal: revisão
Joana Ferreira1, Sandrina Braga, Celso Carrilho, João Correia Simões, Amílcar Mesquita
1Serviço de Angiologia e Cirurgia Vascular — Hospital da Senhora da Oliveira Guimarães EPE
Autor para correspondência
ABSTRACT
The presence of abdominal or back pain, weight loss and elevated erythrocyte sedimentation rate is characteristic of the inflammatory abdominal aortic aneurysms. Low-grade fever is sometimes identified. Other inflammatory markers, such as white blood cell count, C reactive protein, anti-nuclear antibody and IgG4 levels can be elevated. Computed tomography is the exam of choice, showing characteristically the mantle sign. Steroids improve the symptomatology and reduce inflammation, but do not treat the aneurysm. The choice of interventions: open surgical repair or endovascular aneurysm repair can be considered according to the available expertise, patient risk factors, anatomic aneurysm criteria and presence of hydronephrosis.
Keywords: Inflammatory abdominal aortic aneurysm; open surgical repair; endovascular aneurysm repair.
RESUMO
O aneurisma inflamatório da aorta abdominal caracteriza-se por dor lombar ou abdominal, perda de peso e aumento da velocidade de sedimentação. Frequentemente o doente apresenta febre e leucocitose. Outros marcadores inflamatórios poderão estar aumentados como a PCR, os anticorpos-anti-nucleares e o IgG4. A tomografia computorizada é o exame de eleição, apresentando tipicamente o “sinal do manto”. Os esteroides melhoram a sintomatologia, reduzindo a inflamação, mas não corrigem o aneurisma. A decisão, entre intervenção cirúrgica ou endovascular deve ser considerada de acordo com a experiencia do centro, os fatores de risco do doente, as características anatómicas do aneurisma e a presença de hidronefrose.
Palavras-chave: Aneurisma inflamatório da aorta abdominal; intervenção cirúrgica; intervenção endovascular.
INTRODUCTION
Inflammatory abdominal aortic aneurysm (IAAA) is an infrequent pathology, representing 2.5% to 10% of all abdominal aortic aneurysms (AAA). IAAA have peculiar characteristics, which can determine its treatment, particularly, the choice between endovascular or surgical repair. The objective of this paper is to review the published information about IAAA, discuss the treatment options, its advantages and disadvantages.
MATERIAL AND METHODS
A research on MEDLINE® database (http://www.ncbi.nlm.nih.gov/pubmed/) was performed with the words “inflammatory abdominal aortic aneurysm”. Complete articles written in english language and published from January 1997 to April 2017 were selected. From this research, according to the article title and summary, 24 articles were read. Seven were excluded because they did not provide new information and were not included in the references. The figures in this revision article were from the patients treated at the authors' institutions(3).
RESULTS AND DISCUSSION
INFLAMMATORY ABDOMINAL AORTIC ANEURYSM
DEFINITION
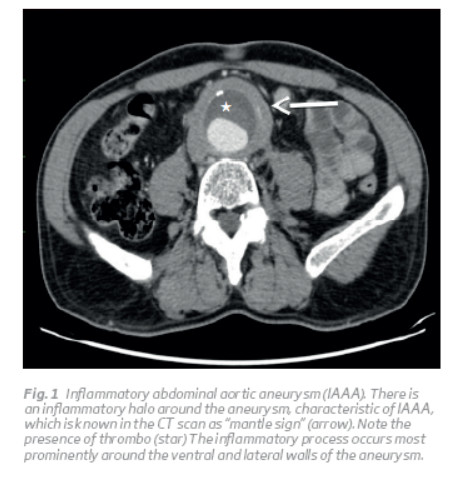
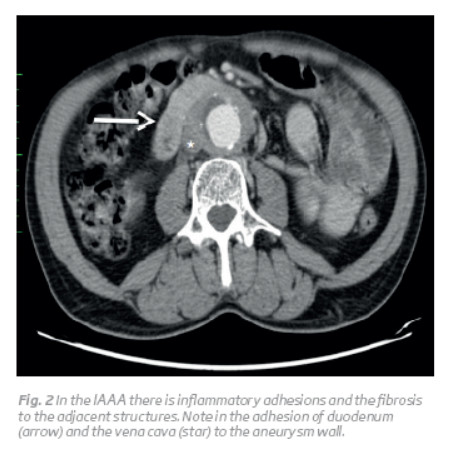
AAA is a focal and permanent dilatation of the aorta, with a diameter greater than 1.5 times the normal dimension of the aorta at the level of the renal arteries(1,2). The normal size of the abdominal aorta varies with age, sex, race and body size, measuring on average 2 cm at the renal level. In this way, a practical working definition of AAA is an aorta with a diameter greater than 3 cm(2). In IAAAs, the aorta is aneurysmatic and surrounded by inflammation, with stiffening of the adjacent tissues (Fig. 1)1. There is perianeurysmal fibrosis and inflammatory adhesions entrapping the surrounding structures 1,2,3. The structures involved in the inflammatory process are duodenum (97–100%), inferior vena cava (63–70%), left renal vein (48–51%), ureters (20–44%), small bowel (20%), and sigmoid colon (5–20%) (Fig. 2 and 3)(3).
NATURAL HISTORY
The natural history of the AAA and of the IAAA is progressive enlargement and rupture(3). The rate of rupture is proportional to the diameter of the aneurysm, and the risk is especially high when the diameter exceeds 5.5 cm(2). Although this risk is considered to be lower in the IAAA compared to the non-IAAA(3,4).
CHARACTERISTICS
The IAAA has particular clinical, anatomic, histologic and epidemiologic features, which differentiate it from the non-IAAA.
The characteristics of the IAAA are: thickening of the aneurysm wall; fibrosis of the retroperitoneum and adherence of the adjacent structures to the anterior aneurysm wall (Fig. 2 and 3)(2). The posterior wall of the aorta is usually spared in inflammatory process and perianeurysmal fibrosis occurs most prominently around the ventral and lateral walls of the aneurysm (for reasons that are not known) (Fig. 1)(2).
Histopathologically there is adventitia expantion with inflammatory reaction, and this characteristic distinguishes IAAA from the non-IAAA(2). The adventitia is filled with plasma cells, lymphocytes (mostly B cells and smaller numbers of CD4 T cells), macrophages and infrequently eosinophils and neutrophils2. It is observed an up-regulation of inflammatory cytokines, including interleukin 2, interleukin 4, interleukin 1α , and adhesion molecules(2).
Elevated levels of immunoglobulin G4 (IgG4) have been associated with inflammatory aneurysm, and such levels may be useful in the diagnosis(5).
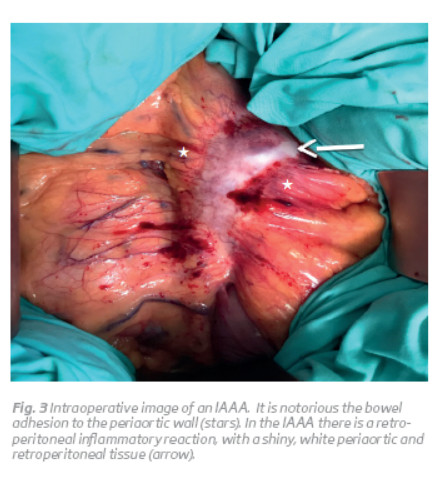
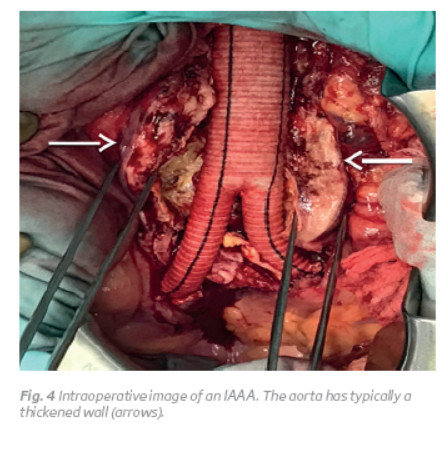
The inflammatory characteristics can be identified intraoperatively, because the aorta has typically a thickened wall, measuring from 0.5 to 3.0 cm, and there is a retroperitoneal inflammatory reaction, with a shiny, white periaortic and retroperitoneal tissue (Fig. 3 and 4)(1,3). Imagiologically, on abdominal computed tomography (CT) scans, there is a typical the 'mantle sign' (Fig. 1)(1).
Another interestingly feature is the diagnose age. The IAAA are diagnosed 5 to 10 years earlier than the mean age of patients with non-IAAAs(2,3).
Beside this, the percentage of smoking patients, males and patients with a positive family history is higher in IAAA compared to the non-IAAA(2,3,6): the smokers approach 100%; the male-to-female ratio, ranges from 6:1 to 30:1 and the percentage of patient with a positive family history is 17% (compared with patients with non-IAAA — 1.7%)(2,3,6).
The IAAA is almost always symptomatic with back pain, low-grade fever, or weight loss4. The serum inflammatory markers and the erythrocyte sedimentation rate are elevated(7).
DIFFERENTIAL DIAGNOSIS
Differential diagnosis with aortic rupture is mandatory, because the patients complaints about abdominal or back pain and CT scans images may mimic rupture(1).
PREVALENCE
Most large series indicate that the IAAA is seen in 2.5% to 10% of all cases of AAA(2,8).
ETIOLOGY AND PATHOGENESIS
Investigators have proposed different theories to explain the inflammatory process but it is not completely understood1. The etiology of AAA (inflammatory and non-inflammatory) is probably multifactorial(3). It is likely that IAAA is the extreme end of an inflammatory process also identified in the non-IAAA(3). This means that aneurysm formation could be the initial event and the inflammation is a secondary change(4). In fact, the adventitia is infiltrated with inflammatory cells in AAA(3). The difference between IAAA and non-IAAA is the intensity and extent of the inflammatory process, suggesting that they are the same disease, differing only in the progression of the inflammation(3).
It is believed that a non identified antigen is presented to the aortic wall, provoking a immunologic response characterized by aortic-wall infiltrating macrophages, T lymphocytes, and B lymphocytes(3). They activate proteolytic activity with the production of cytokines(3). This proteolytic activity leads to increased turnover in the aortic wall proteins and aneurysm formation(3).
As previously stated the precise antigen target has not been defined(2). One hypothesis is that chemical factors, such as tobacco, or a lipid, or a product of lipid oxidation found in an atherosclerotic aorta can increase the inflammatory process, predisposing to the development of IAAA(2,3). Other hypothesis is an infective antigen, a vírus such as cytomegalovirus or bacteria, but bacterial cultures of the aneurysm wall and serologic tests for syphilis consistently have been negative(1,2,3). The genetic predisposes certain persons to develop the AAA(3).
In summary, some authors suggested that atherosclerotic aneurysm formation is the preceding event and that the inflammatory response is a secondary change. However other reports described the possible involvement of an autoimmune reaction. Patients with IAAA sometimes show serological autoimmune abnormalities; such as, the presence of autoantibodies(8).
Based on his immunological studies on inflammation, Kasashima proposed a classification of IAAAs as immunoglobulin IgG4-related and IgG4-non-related, thus emphasising an immunological role in the development of the disease. Evidence of a genetic predisposition has also been demonstrated, but ultimately, the etiology may be multifactorial(1).
SYMPTOMS
Most of the patients are symptomatic (65% to 90%)(1,3,8). Back, abdominal, and flank pain, with or without a pulsatile abdominal mass are the most common finding(1,2,3).
Fever, weight loss and anorexia have also been reported (20 to 41%)(1,2,3,8).
Duodenal or inferior vena caval obstruction and ureteral colic might be present due to retroperitoneal fibrosis(3,8).
Clinical presentation with acute abdominal pain and circulatory collapse is rare, because the lifetime risk of rupture is less than 5%(2).
In patients with AAA, the triad of abdominal or back pain, weight loss, and an elevated erythrocyte sedimentation rate is highly characteristic of IAAA(3).
PHYSICAL FINDINGS
The most common finding is abdominal tenderness with or without a pulsatile abdominal mass, but is present in approximately one third of patients(2).
ASSOCIATED COMORBIDITIES
The presence of other aneurysm besides the abdominal aorta is common, but are not usually inflammatory in gross appearance, except in the thoracic aorta(1,3,4). (Fig. 5). Obtain imaging of the entire aorta, and from the limb arteries is mandatory(4).
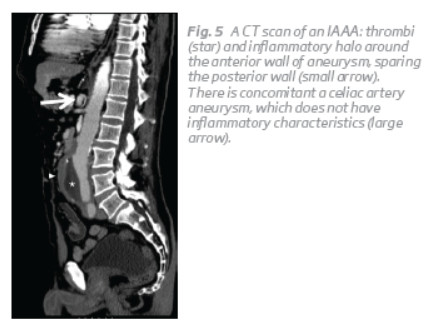
Arterial hypertension, coronary artery disease, arterial occlusive disease are frequent comorbidities associated with both IAAA and non-IAAA(3).
DIAGNOSTIC TEST
Ultrasound can diagnose an IAAA. The feature of this aneurysm is the presence of a sonolucent halo, surrounding the aneurysm(3). However accordingly to literature the sensibility of ultrasound varies between 0% to 13.5%(3,8). Rasmussen stated that reviewing these ultrasounds, the findings suggestive of an IAAA were identified in 60% of patients(3). The sonolento halo is sometimes misdiagnosed as an AAA ruptured.
The ultrasound sensibility in identifying IAAA is inferior to that of CT scan(3). The sensibility CT scan is between 80–90%(8).
CT scan and magnetic resonance can both demonstrate the characteristic soft tissue thickening around the aneurysm, which is known as “mantle sign” (Fig. 1 and 5)(1,3,8).
Magnetic resonance imaging also avoids the administration of nephrotoxic intravenous contrast material and radiation exposure(2). The periaortic mass in inflammatory AAA is hypointense on T1-weighted images, but hyperintense on T2-weighted images(2). The periaortic of inflammation enhances with administration of intravenous gadolinium(2). Some physicians, emphasize that magnetic resonance provides less information about the ureters, reserving magnetic resonance for patients who have renal insufficiency and thus preferring CT scan(2).
In conclusion the CT scan should be the exam of choice(8).
In the IAAA the fluorodeoxyglucose positron emission tomography scanning can identify active periaortic inflammation(1).
The laboratory inflammatory markers can be elevated, such as erythrocyte sedimentation rate, C reactive protein, white blood cell count, antinuclear antibody and IgG4 levels(1,3).
THERAPY
MEDICAL THERAPY
The medical management includes minimizing atherosclerotic risk factors, with smoking cessation, hypertension and hyperlipidemia control(4,7).
Some authors advice steroid treatment, however its efficacy on retroperitoneal fibrosis is controversial(9). It can improve the symptoms, signs and reduce the inflammatory ring of IAAA, consequently decreasing the surgery technique difficulty(4). They can avoid the inflammation progression and its recurrence after surgery(4). Methotrexate, cyclophosphamide and azathioprine, have also been used, although without scientific recommendations(4). However corticosteroids do not change the natural history of the IAAA and the aneurysm correction should be performed(3).
SURGICAL VERSUS ENDOVASCULAR THERAPY
There are no randomised controlled trials comparing open surgical repair (OSR) with endovascular aneurysm repair (EVAR) for IAAA(6). A systematic review performed by Paravastu concluded that there was no significant difference in 30-day mortality (2.4% with EVAR vs. 6.2% with open repair)(1,6). Paravastu also stated that both OSR and EVAR can be safely performed in IAAA and that the benefit of EVAR over OSR for IAAA will be similar to that for non-IAAA(6). However in IAAA, besides rupture prevention, the treatment should decrease the rate of periaortic fibrosis and revert the hydronephrosis, if it is present(1). The studies showed that periaortic fibrosis regressed with both OSR and EVAR, however with diferente velocities(6).
SURGICAL THERAPY
Due to the inflammatory nature of the aneurysm and consequente retroperitoneal fibrosis the IAAA surgical repair leads to injury to the adjacent structures and increased bleeding(5,7). Surgery can be complicated by enterotomies, mainly of the duodenum and injuries of the ureters and the vena cava, raising the operative mortality(3).
As a result, the morbidity after OSR as reported in EUROSTAR registry is typically high(15). This fact in part from an unselected patient population, some of them treated as an emergency because of a symptomatic AAA, or due to the CT image of an inflammatory ring interpreted as aneurysm rupture(7,15).
In order to minimize the complications, the authors recommend modified approaches, such as avoiding the duodenum dissection, obtaining proximal and distal control of the aneurysm “with as little dissection” as possible, if needed performing a supraceliac cross-clamping of the aorta(3,15). Some authors also recommend retroperitoneal approach, because it is thought to reduce the risk for duodenal, left renal vein and inferior vena cava injury(15). Despite the many technical difficulties, surgical results have greatly improved over the last 35 years(2). With the technique of limited dissection, the operative mortality rates is 3% to 4%, matching those of non IAAA(3).
With OSR clinical symptoms (abdominal pain, malaise, and weight loss) reversed in 93% of the patients(3). The surgery decreases the peri-aortic fibrosis and the debridement of abdominal adjacent organs decreases the symptoms(1). The fibrosis frequently entraps one or both ureteres, causing hydronephrosis(1). The reversion of hydronephrosis is particularly importante and occurs in a great extension in OSR compared to EVAR(1). In the meta-analysis performed by Paravastu, he stated that at one year, peri-aortic fibrosis regressed in 73% in the OSR group compared to 65% of the EVAR group(6). 45 patients undergoing OSR and in 29 submitted to EVAR were found to have preoperative hydronephrosis(6). This regressed postoperatively in 69% and 38% respectively(6). The regression of hydronephrosis is observed even after excluding the patients who underwent ureterolysis(6). However hydronephrosis and inflammation can progress even after IAAA surgical treatment(6). Paravastu documented inflammation and hydronephrosis progression in 1% and 9% of patients undergoing surgery(6). New-onset hydronephrosis developed in 6% undergoing OSR(6).
Several authors defend that OSR is the preferred therapy in cases involving inflammatory aneurysms complicated by hydronephrosis(5).
Due to the variable and unpredictable risk of ureteral entrapment, and in order to avoid hydronephrosis, some authors advocate the preoperative insertion of ureteral stents(10,11). They argue also, that the stents would help identifying the ureters intraoperatively, minimizing iatrogenic injuries(10). However the majority of authors are against routine ureteral stenting. They state that the risk of renal function impairment is low (about 15%), and even in this case, an emergent urological procedure, such as a temporary percutaneous nephrostomy may be performed(10,12). Moreover the ureter stent insertion is not free of complications, such as migration, fracture, occlusion, encrustation, stone formation and deployment of a stent in dense fibrosis can lead to ureteral injury(10,13). However if a stent is needed, monthly clinical, imagiological and laboratorial monitorization is mandatory and timely removal is essential(13,14).
Another interesting data is related with the higher rate of pseudoaneurysms after the surgical repair of IAAA, compared with non-IAAA(8). Nuellari in a serie of 35 IAAA reported: a femoral pseudoaneurysm; an aortic pseudoaneurysm 8 months after surgery and 3 pseudoaneurysm from 2 to 6 years after the operation(8). The overall pseudoaneurysm formation rate was 14.2%(8). The authors considered that the IAAA have special characteristics predisposing to the development of this complication(8). The inflammatory cells and endarteritis obliterans with subsequent ischemic aortic wall damage, as well as elastin depletion and collagen alterations could induce arterial weakness leading to pseudoaneurysmal degeneration(8).
ENDOVASCULAR THERAPY
Due to the technical difficulties of the OSR, in anatomically suitable IAAAs, EVAR seems an attractive option(1,7).
Stone defends that EVAR should be considered the first-line therapy in IAAAs(16).But the published results failed to demonstrate the regression of fibrosis in all patients who performed EVAR or even documented its worsening(6,7,15,17).
The rate of fibrosis regression after EVAR varies according to the published series. Absence of peri-aortic fibrosis regression was reported in 33–55%(15,17). The meta-analysis performed by Paravastu showed a lack of regression in 31% and progression in 4% after EVAR(6).
In the same way, regression of hydronephrosis after EVAR could be a slow process and is reported in less than half of cases (in 38% of EVAR patients)(6,9). De novo hydronephrosis development occurs in 2% to 20% of IAAA patients treated with EVAR, with the risk for renal atrophy (Fig. 6)(9,15). Therefore, it is unclear whether EVAR has any beneficial effects on preoperative hydronephrosis(5). It is suggested, that is advisable to perform additional, urological treatment and imagiologic surveillance to evaluate hydronephrosis(9,15). A systematic review showed that steroids can decrease the incidence of deterioration of post-operative hydronephrosis after EVAR from 25% without steroids to 11% with steroid use(6).
These results stated above could be explained by the inflammatory stimuli associated with the implantation of an endoprosthesis, which may be an etiological factors in the progression of peri-aortic fibrosis, despite proper treatment of the aneurysms(5). Moreover, it is thought that the development of thrombi in the intrasac is a possible trigger of peri-aortic inflammatory by inducing an autoimmune response(5).
This inflammatory nature of the aneurysm and fibrosis progression can also justify the high rate of secondary intervention after EVAR for IAAA, compared with the rate of interventions to non-IAAA(6). The EUROSTAR registry showed that IAAAs treated by EVAR have a higher incidence of stent stenosis compared to non-IAAA: 3.9% versus 0.3%, respectively(6). Secondary intervention rate after EVAR in IAAA was 22% which is higher compared to the reports of EVAR trial 1 (type II endoleak in 3% and type III endoleak in 1% were observed)(6). Therefore, several authors concluded that EVAR in IAAA must be carefully weighed(9).
Beside these facts, the EUROSTAR reported that the morbidity of EVAR for IAAAs is the same as the reported to treat atherosclerotic aneurysm(15).
EVAR of IAAAs is feasible, effective and with decreased procedure duration, transfusion needs, hospitalization, low morbidity and mortality and an acceptable re-intervention rate(1,15). However due to the risk of a peri-aneurysmatic fibrosis progression and renal impairment, at a minimum, close abdominal ultrasound surveillance to evaluate hydronephrosis seems indicated(9,15).
FOLLOW-UP
The patients with IAAAs treated with OSR or EVAR require close clinical and imagiologic surveillance as there appears to be a significant risk for anastomotic pseudoaneurysm formation, hydronephrosis progression and stent graft stenosis in follow-up(5).
CONCLUSION
The presence of abdominal or back pain, weight loss and elevated erythrocyte sedimentation rate is characteristic of the IAAA. Low-grade fever is sometimes present. Other inflammatory markers, such as white blood cell count, C reactive protein, anti-nuclear antibody and IgG4 levels can be elevated. Computed tomography is the examen of choice, showing characteristically the mantle sign. The steroids improves the symptomatology and reduces the inflammation, but does not treat the aneurysm. The intervention: OSR or EVAR can be considered according to the available expertise, patient risk factos, anatomic aneurysm criterias and presence of hydronephrosis. In both options strategy is the key.
REFERENCES
1. Capoccia L, Riambau V. Endovascular repair versus open repair for inflammatory abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015 Apr 16;(4). [ Links ]
2.Hellmann DB1, Grand DJ, Freischlag JA Inflammatory abdominal aortic aneurysm. JAMA. 2007 Jan 24;297(4):395–400. [ Links ]
3. Rasmussen TE, Hallett JW Jr. Inflammatory aortic aneurysms. A clinical review with new perspectives in pathogenesis. Ann Surg. 1997 Feb;225(2):155–64. [ Links ]
4. Ketha SS, Warrington KJ, McPhail IR. Inflammatory abdominal aortic aneurysm: a case report and review of literature. Vasc Endovascular Surg. 2014 Jan;48(1):65–9. [ Links ]
Correio eletrónico: joana222@hotmail.com (J. Ferreira).
Recebido a:23-05-2017;
Aceite a 25-10-2017;














