Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Angiologia e Cirurgia Vascular
versão impressa ISSN 1646-706X
Angiol Cir Vasc vol.14 no.4 Lisboa dez. 2018
ARTIGO ORIGINAL
Intraoperative anticoagulation monitorization in vascular surgery - Does a blind dosis fits all?
Monitorização intraoperatória da anticoagulação em cirurgia vascular - Uma dose cega serve para todos?
Nuno Henriques Coelho1, Raquel Laranja Pontes2, Rita Silva2, Victor Martins1, Cármen Oliveira2, Jacinta Campos1, Pedro Sousa1, Andreia Coelho1, Rita Augusto1, Carolina Semião1, Evelise Pinto1, João Ribeiro1, Alexandra Canedo1, Carla Bentes2
1Serviço de Angiologia e Cirurgia Vascular, Centro Hospitalar Vila Nova de Gaia/Espinho
2Serviço de Anestesiologia, Centro Hospitalar Vila Nova de Gaia/Espinho
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Introduction: Unfractionated heparin (UFH) has been used for decades to prevent thrombotic events during vascular surgery. Although it is known that UFH has a complex and nonlinear pharmacokinetics, with great individual variability, anticoagulation monitorization in vascular surgery is not routine and a standard empirical dose is often used. Activated clotting time (ACT) has been shown to be a simple, reliable and inexpensive way to monitor UFH anticoagulant effect, being routinely used during cardiac surgery. However, heparinisation remains a dilemma in vascular surgery and few studies emphasized the role of anticoagulation monitoring in this setting.
Objectives: To investigate whether a fixed heparin dose of 5000 IU in arterial vascular surgery results in adequate and homogeneous heparinisation in all patients. Secondary endpoints: to identify preoperative factors for heparin response, intraoperative events and outcomes.
Methods: This observational prospective pilot study included 30 consecutive patients undergoing arterial vascular surgery. ACT monitoring was performed before clamping and at 3, 30 and 60 minutes after 5000 IU UFH bolus. Preoperative and intraoperative data were also accessed. A target ACT of = 200 s was set, taking in account of the lowest ACT value admitted by vascular surgery recommendations.
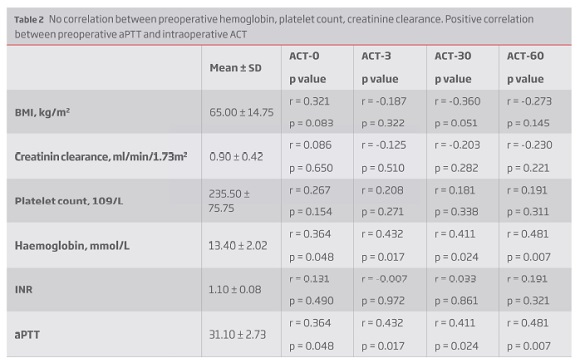
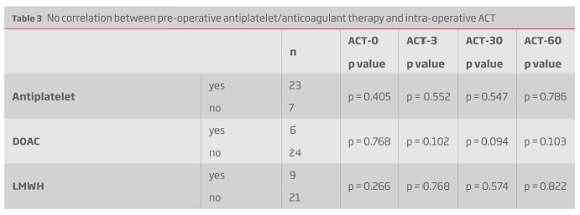
Results: The average ACT value increased to 210.20 ± 28.82 s (1.61 ± 0.25 times vs baseline) 3 minutes after bolus, then declined to 191.60 ± 21.86 s and 173.4 ± 21.37 s after 30 and 60 minutes, respectively. Three minutes after UFH bolus, 53% patients had ACT = 200 s, decreasing to one third and 7% at 30 and 60 minutes, respectively. Even when weight-based, a correlation between heparin dose per kilogram and ACT change was not found (r = 0.187; p = 0.322). There was also no correlation between ACT values and preoperative hemoglobin, platelet count, creatinine clearance or INR. There was a positive correlation between preoperative aPTT and intraoperative ACT measurements (r = 0.432; p = 0.017). There was no difference between ACT values and previous antithrombotic/anticoagulant therapy and between intraoperative ACT and intraoperative blood loss.
Conclusions: This study confirms that administrating a fixed or even a weight-based heparinisation is insufficient to provide consistent anticoagulation levels in all patients. Perioperative anticoagulation should be monitored and ACT-based. Larger clinical RCT's are warranted.
Keywords: Unfractionated heparin, Heparinization monitoring, Activated clotting time, Anticoagulation monitorization, Vascular surgery
RESUMO
Introdução: A heparina não fracionada (HNF) tem sido usada há décadas na Cirurgia Vascular como medida para prevenção de fenómenos tromboembólicos. A sua farmacocinética complexa e não linear, associada a grande variabilidade individual, está amplamente documentada. Contudo, a monitorização da anticoagulação não é realizada por rotina em Cirurgia Vascular e uma dose standard é muitas vezes utilizada. O activated clotting time (ACT) tem mostrado ser uma forma simples, confiável e económica de monitorizar o efeito da HNF, sendo usado por rotina durante a cirurgia cardíaca. Contudo, a monitorização da heparinização mantém-se um dilema em Cirurgia Vascular sendo escassos os estudos que enfatizam a importância da monitorização.
Objetivos: analisar se uma dose fixa de HNF (5000 UI) usada durante cirurgia arterial resulta numa heparinização adequada e homogénea em todos os doentes. Objetivos secundários: identificar fatores pré-operatórios, eventos intraoperatórios e outcomes que se correlacionem com o valor de ACT obtido.
Métodos: Este estudo piloto observacional e prospetivo incluiu 30 doentes consecutivos sujeitos a cirurgia arterial. A monitorização do efeito da HNF foi realizada através de medições seriadas do ACT (antes da clampagem e 3, 30 e 60 minutos após o bólus de heparina). Dados pré-, intra- e pós-operatórios foram também analisados. Dada a inexistência de guidelines ou recomendações concordantes quanto ao valor de ACT adequado para cirurgia arterial, foi tido em conta o menor valor referido nas diversas recomendações analisadas (ACT = 200 s).
Resultados: Aos 3 minutos constatou-se um ACT médio de 210.20 ± 28.82 s (1.61 ± 0.25 vezes o ACT basal). Após esta medição verificou-se um declínio para os 191.60 ± 21.86 s e 173.4 ± 21.37 s após 30 e 60 minutos, respetivamente. Três minutos após o bólus de heparina, apenas 53% dos doentes atingiram o ACT alvo, diminuindo esta percentagem para um terço aos 30 minutos e apenas 7% aos 60 minutos. Mesmo quando ajustada ao peso, não se verificou uma correlação estatisticamente significativa entre a dose de heparina (UI/Kg) e o ACT medido após o bólus (r = 0.187; p = 0.322). Também não se constatou uma correlação entre os valores de ACT obtidos e os valores pré-operatórios de hemoglobina, plaquetas, clearance de creatinina e INR. Verificou-se uma correlação positiva entre o valor de aPTT pré-intervenção e os valores de ACT (r = 0.432; p = 0.017). Não foi constatada diferença entre os valores de ACT obtidos e a toma prévia de antiagregantes e anticoagulantes, bem como não houve diferença entre os valores de ACT alcançados e as perdas sanguíneas intraoperatórias.
Conclusão: Este estudo vem demonstrar que, quer um regime de heparinização baseado numa dose fixa quer baseado no peso do doente, são insuficientes para proporcionar uma anticoagulação adequada sugerindo que a heparinização intraoperatória deve ser monitorizada e guiada pelos valores de ACT obtidos. São necessários mais estudos para definir as reais implicações desta monitorização, os seus benefícios e as ações necessárias perante um determinado valor de ACT obtido.
Palavras-chave: Heparina não fracionada, Monitorização intraoperatória da anticoagulação, Activated clotting time, Cirurgia vascular
Introduction
Since Murray first used heparin for arterial reconstruction in 1940(1), unfractionated heparin (UFH) has been the anticoagulant of choice to prevent thromboembolic events during open and endhovascular arterial sugery(2). It is known that UFH is a heterogeneous molecule, with low volume distribution, having a complex and unpredictable pharmacokinetics, with wide individual dose-response and elimination variability(3). UFH response is even more unpredictable in patients with already deregulated coagulation cascade, as it is the case of the vascular surgery patients (often operated under antiaggregation and/or anticoagulation). This individual variability can either lead to bleeding or thrombotic events increasing operation time, blood loss, transfusion requirements and re-interventions, enhancing infectious complications and morbimortality. Despite of that, anticoagulation monitorization in non-cardiac vascular interventions is not routine and frequently a standardized bolus of 5000 U UFH is used(4). Activated clotting time (ACT) has been shown to be a simple, reliable and inexpensive way to monitor UFH anticoagulant effect, with no need for special training. ACT based UFH monitoring is routine during cardiac interventions and has well defined targets. However, heparinisation remains a dilemma in non-cardiac vascular surgery, with different heparin dosing algorithms (fixed dose, weight-based, redosing schedules, protamine reversal), frequently without any monitorization and with no optimal ACT target defined(2,4,6-8). Therefore, the objective of this pilot study was to investigate if a fixed heparin dosing regimen resulted in adequate and homogeneous anticoagulation in all patients. Potential pre-operative factors for heparin response were also studied as well as intra-operative events and outcomes with relation to ACT values.
Methods
Study population and design
This observational prospective pilot study resulted from a multidisciplinary cooperation between Vascular Surgery and Anesthetic Department of our institution and the protocol was approved by the Local Institution Ethics Committee. Patients older than 18 years, able to sign or delegate a representative, undergoing open arterial procedure requiring heparinization were eligible. Patients with history of coagulation disorders, glomerular filtration rate < 30 ml/min and emergency or early re-operations were excluded. All eligible patients provided written informed consent and patient inclusion started in December 2017, ending in March 2018. Patient's demographics, preoperative laboratory data, surgical events and clinical outcomes were accessed. Intraoperative blood samples for ACT measurements were collected from an arterial line, after rejecting heparinized saline solution from the system. All samples were handled by the same operator and processed in the same device (I-STAT, Abbott Laboratories, Illinois, US). Basal ACT (ACT-0) was measured after anesthesia induction. Then all patients received a fixed intravenous bolus of 5000 IU UFH. Another three ACT measurements were performed thereafter: at 3, 30 and 60 minutes (ACT-3, ACT-30 and ACT-60, respectively). ACT elevation was expressed both as actual ACT value and ACT ratio. As there is no optimal ACT value defined for non-cardiac vascular interventions, a target value of 200 s was selected, considering the scarce data that show that a minimum of 200 s or a two-fold extension of baseline ACT could be safe in preventing thromboembolic events(9,4).
Statistical analysis
Statistical analysis was performed using the SPSS statistical software package 23.0 (IBM, New York, NY, USA). Continuous, normally distributed variables were expressed as mean ± standard deviation and analyzed using a t-test. Skewed and ordinal data were shown as median with interquartile range (IQR) and analyzed using Mann-Whitney test. Categorical variables were expressed as frequencies and analyzed using the chi-square test or the Fisher's exact test, as appropriate. Correlations between continuous variables were accessed using the Spearman correlation coefficient. A p value of 0.05 was considered to be statistically significant.
Results
Patient characteristics
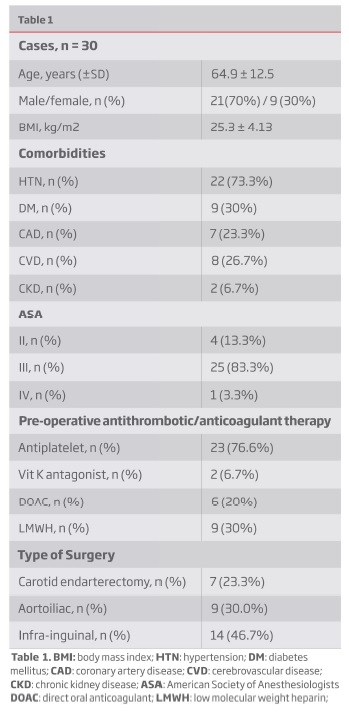
Thirty patients undergoing open arterial non-cardiac vascular surgery (carotid n=7; aortoiliac n=9; infra-inguinal n=14) were included. The demographic characteristics of the study group are summarized in Table 1. The average age was 64.9 ± 12.5 years, with 70% males and a high prevalence of hypertension (73.3%), diabetes (30%), coronary artery disease and cerebrovascular disease (23.3% and 26.7%, respectively). The periprocedural use of one or more antiplatelet agents was also remarkable.
Anticoagulation monitoring
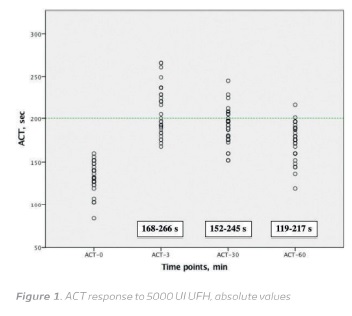
The baseline ACT values were 131.90 ± 17.30 (range, 83-160 s). Three minutes after 5000 U UFH bolus the average ACT increased to 210.20 ± 28.82 s (range, 168-266 s) corresponding to a 1.61 ± 0.25 times baseline increase (range, 1.23-2.36 times baseline). After 30 minutes ACT declined to 191.60 ± 21.86 s (range, 152-245 s) and to 173.40 ± 21.37 s (range, 119-217 s), after 60 minutes (Figure 1 and 2).
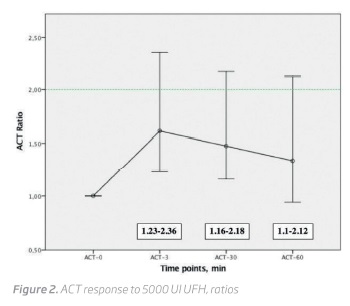
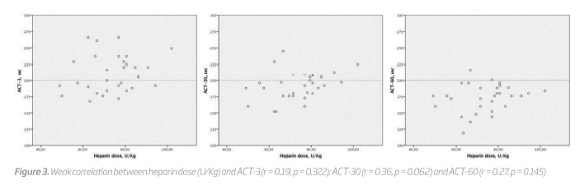
Although all patients received 5000 UI UFH, the dose received per kilogram varied between 49.02 and 102.04 U/kg (mean 73.70 ± 12.44 U/kg). However, even when weight-adjusted, a correlation between heparin dose per kilogram and ACT change was not found at all measurement points (r = 0.19, p = 0.322, at 3 minutes; r = 0.36, p = 0.062, at 30 minutes and r = 0.27, p = 0.145, at 60 minutes) (Figure 3).
There was also no correlation between preoperative hemoglobin, platelet count, creatinine clearance and ACT values neither between ACT measurements and intraoperative blood loss. There was however a positive correlation between preoperative aPTT and intraoperative ACT measurements (Table 2). There was no difference between preoperative antithrombotic/anticoagulant therapy and ACT values (Table 3).
Peri-and post-operative complications
After carotid endarterectomy, a patient developed a cervical hematoma requiring immediate surgical re-exploration (perioperatively: ATC-0 = 144 s; ACT-3 = 266 s; ACT-30 = 222 s and ACT-60 = 217 s).
Discussion
UFH is extensively advised and used in all types of vascular procedures (open and endovascular) as periprocedural antithrombotic prophylaxis, despite its known variable response. In contrast with cardiac interventions, the measurement of actual anticoagulation has not been widely incorporated as standard of care during non-cardiac vascular interventions. Recent reports state that only a minority of vascular surgeons requests intraoperative monitoring(2). Most procedures undergo without monitorization, with potential arm to the patient. Vascular surgery patients are a high-risk cohort with multiple comorbidities. Therefore, it is important to minimize bleeding risk and, at the same time, to maintain adequate anticoagulation level. The present study demonstrates a wide variation in the response to heparin and suggests that neither a fixed or a weight-based regimen provide adequate and homogeneous anticoagulation and this may influence patient's surgical risk. In our population, as previously described, 3 minutes after heparin bolus nearly only half of the patients had an adequate ACT value, decreasing to one third after 30 minutes. After 60 minutes, we found only two patients with an ACT > 200 s. These results are in agreement with the current literature (like the MANCO study) and suggest that a significant percentage of vascular interventions undergo without appropriate anticoagulation. The multifactorial origin of surgical complications, along with the small sample size, makes impossible to take statistically significant conclusions about intraoperative ACT monitorization and adverse events. However, it is worthy to note that the patient who developed a cervical hematoma after carotid endarterectomy presented with an ACT value of 217 s, one hour after heparin bolus (around the time of closure). Recent studies, state that the use of protamine at the time of closure of a carotid endarterectomy is associated with a reduction of the incidence of cervical hematomas without increasing major thrombotic outcomes(10,11). Additionally, regular ACT monitoring is suggested as a guide to selectively evaluate the need of heparin reversion with protamine in the same type of intervention(12-;14). Extensive research has been performed in interventional and cardiac surgery about perioperative anticoagulation. However, different hemodynamics, distinct target vessels and different procedures demand specific answers. Larger clinical studies are warranted to further define what should be the optimal ACT range in vascular surgery procedures, to understand its consequences and what actions should be taken when faced with different ACT values.
Conclusion
There is a multifactorial nature for surgical complications, however the non-uniform response to heparin may be one factor to take in account. This pilot study intends to be a starting point for a larger study population in our institution, already in progress. These initial results (in accordance with recent publications) reinforce that empiric heparinization, even when weight-based, is insufficient to provide consistent anticoagulation levels in all patients, suggesting that it should be monitored and ACT-based.
REFERENCES
1. Murray, g. Heparin in surgical treatment of blood vessels. Arch. Surg. 40, 307 (1940). [ Links ]
2. Wiersema, A. M. et al. Periprocedural prophylactic antithrombotic strategies in interventional radiology: Current practice in the netherlands and comparison with the United Kingdom. Cardiovasc. Intervent. Radiol. 36, 1477-1492 (2013). [ Links ]
3. de Swart, C. A., Nijmeyer, B., Roelofs, J. M. & Sixma, J. J. Kinetics of intravenously administered heparin in normal humans. Blood 60, 1251-8 (1982). [ Links ]
4. Veerhoek, D. et al. Individual Differences in Heparin Sensitivity and Their Effect on Heparin Anticoagulation During Arterial Vascular Surgery. Eur. J. Vasc. Endovasc. Surg. 54, 534-541 (2017). [ Links ]
5. Wiersema, A. M. et al. The Use of Heparin during Endovascular Peripheral Arterial Interventions: A Synopsis. Scientifica (Cairo). 2016, (2016). [ Links ]
6. Kasapis, C. et al. Defining the Optimal Degree of Heparin Anticoagulation for Peripheral Vascular Interventions: Insight From a Large, Regional, Multicenter Registry. Circ. Cardiovasc. Interv. 3, 593-601 (2010). [ Links ]
7. Assadian, A. et al. Antithrombotic strategies in vascular surgery: Evidence and practice. Eur. J. Vasc. Endovasc. Surg. 29, 516-521 (2005). [ Links ]
8. Moussa, O., Jonker, L. & Joseph, T. Marked Variation in Venous Thromboprophylaxis Management for Abdominal Aortic Aneurysm Repair; Results of Survey Amongst Vascular Surgeons in the United Kingdom. Eur. J. Vasc. Endovasc. Surg. 42, 591-595 (2011). [ Links ]
9. Goldhammer, J. E. & Zimmerman, D. Author s Accepted Manuscript. (2017). doi:10.1053/j.jvca.2017.04.047 [ Links ]
10. Baracchini, C. & Ballotta, E. The Benefit of Heparin Reversal With Protamine During Carotid Endarterectomy. JAMA Surg. 151, 255 (2016). [ Links ]
11. Newhall, K. A., Saunders, E. C., Larson, R. J., Stone, D. H. & Goodney, P. P. Use of Protamine for Anticoagulation During Carotid Endarterectomy. JAMA Surg. 151, 247 (2016). [ Links ]
12. Poisik, A. et al. Safety and efficacy of fixed-dose heparin in carotid endarterectomy. Neurosurgery 45, 434-41; discussion 441-2 (1999). [ Links ]
13. Kakisis, J. D. et al. Protamine Reduces Bleeding Complications without Increasing the Risk of Stroke after Carotid Endarterectomy: A Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 52, 296-307 (2016). [ Links ]
14. De Sousa, A. A., Dellaretti, M. A., Faglioni, W. & Carvalho, G. T. C. Monitoring of activated coagulation time in carotid endarterectomy. Surg. Neurol. 64, 7-10 (2005). [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: nunoc.90@gmail.com (N. Coelho).
Ethical responsabilities
Protection of patients and animals: The authors state that for this investigation no experiments were performed on humans and / or animals.
Confidentiality of the data: The authors state that they have followed the centre ´s established protocols on the publication of patient data.
Right to privacy and informed consent: The authors declare that no patient data is available in this article.
Conflict of interest: The authors declare no potential confict of interest.
Recebido a 02 de julho de 2018
Aceite a 14 de janeiro de 2019