Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Angiologia e Cirurgia Vascular
versão impressa ISSN 1646-706X
Angiol Cir Vasc vol.15 no.2 Lisboa jun. 2019
ARTIGO DE REVISÃO
Non-surgical Treatments of Lymphedema of the Lower Limbs
Tratamento médico do linfedema dos membros inferiors
Francisca Rosas1; Ivone Silva1,2; Rui de Almeida1,2
1Instituto de Ciências Biomédicas Abel Salazar (ICBAS) - Universidade do Porto
2Serviço de Angiologia e Cirurgia Vascular, Centro Hospitalar Universitário do Porto
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Background: LLL is characterized by protein-rich interstitial fluid accumulation due to lymphatic system insufficiency, resulting in progressive non-pitting edema. Primary and secondary lymphedema are distinguished by the absence or presence of an external trigging factor, respectively. Diagnosis is based on clinical examination combined with imaging modalities. The non-surgical approach of LLL is based on preventive measures, lifestyle interventions and life-long compression modalities, aiming to reduce the edema congestion and to improve patient quality of life.
Objectives: The present literature review aims to compile current scientific knowledge on the various domains of the non-surgical treatments of LLL.
Methods: Search was performed in PubMed database, using the following medical subjects heading (MeSH) terms: “lymphedema”, “lower limbs” and “medical treatment”. Research and review articles indexed in the last 10 years and written in English language were selected. Animal experimentation works and single case-reports were excluded. Other materials searched comprised reference books in the area of Vascular Surgery, namely Rutherford Vascular Surgery - 9th Edition, 2018.
Development: The non-surgical treatment of LLL is characterized by a multifaceted approach, including mechanical reduction of limb swelling, alternative medical devices and pharmacological therapy. Complex decongestive therapy is recognized as the standard of treatment and its compression modalities include drainage massages, pneumatic compression, bandaging systems, compression garments and physical exercise. Compression therapies differ in the degree and time of pressure applied and can be performed by healthcare professionals or by the patient himself. The duration of treatment stages varies and it may include hospital and outpatient regimens. Positive treatment outcomes include increased skin elasticity, limb volume reduction, pain relief, increased functional capacity and improved quality of life.
Conclusions: Compression modalities have been shown to be effective in reducing limb volume, increasing tissue elasticity and improving the physical, psychological and aesthetic aspects of patient life. However, sustained volume reductions depend mostly on patient diligence. Intensive and outpatients approaches do not compromise treatment efficacy and high pressures therapies are effective and well tolerated. Current lymphedema non-surgical treatment lacks long-term results and urges more effective therapeutic alternatives.
Keywords: lymphedema, lower limbs, medical treatment
RESUMO
Introdução: O linfedema dos membro inferior é caracterizado pela acumulação de líquido intersticial rico em proteínas consequente a uma insuficiência do sistema linfático, resultando em edema progressivo e não depressível do membro inferior. Há 2 tipos de linfedema primário e secundário. O primeiro sem etiologia conhecida. O diagnóstico é feito através da avaliação clínica em combinação com modalidades de imagem. O tratamento médico do linfedema dos membros inferiores é baseado em medidas preventivas, alterações do estilo de vida e modalidades de compressão contínuas, com o objetivo de reduzir a congestão do edema e melhorar a qualidade de vida dos doentes.
Objetivos: A presente revisão literária tem como objetivo compilar o conhecimento científico atual acerca dos diversos domínios do tratamento médico do linfedema dos membros inferiores.
Metodologia: A pesquisa foi realizada na base de dados do PubMed, utilizando os seguintes termos médicos (MeSH): “lymphedema”, “lower limbs” e “medical treatment”. Foram selecionados artigos de investigação e de revisão indexados nos últimos 10 anos e escritos em inglês. Os projetos de experimentação animal e relatos de caso clínico isolado foram excluídos. Outros materiais pesquisados incluíram livros de referência na área de Cirurgia Vascular, nomeadamente Rutherford Vascular Surgery - 9ª Edição, 2018.
Desenvolvimento: O tratamento médico do linfedema dos membros inferiores é caracterizado por uma abordagem multifacetada, que inclui a redução mecânica do edema dos membros, dispositivos médicos alternativos e terapia farmacológica. A terapia descongestiva complexa é considerada o tratamento padrão e suas modalidades de compressão incluem massagens manuais de drenagem, compressão pneumática, sistemas de bandagem, vestuário de compressão e exercício físico. As terapias de compressão diferem no grau e tempo de pressão aplicados e podem ser administradas por profissionais de saúde ou pelo próprio doente. A duração das fases do tratamento é variável e este pode incluir regimes hospitalares e em ambulatório. Os resultados positivos do tratamento incluem o aumento da elasticidade da pele, a diminuição do volume do membro, a redução da dor, o aumento da capacidade funcional e a melhoria da qualidade de vida.
Conclusões: As modalidades de compressão demonstraram ser eficazes na redução do volume dos membros, aumentando a elasticidade dos tecidos e melhorando os aspetos físicos, psicológicos e estéticos da vida do doente. No entanto, a manutenção das reduções de volume dependem principalmente da aderência ao tratamento por parte do doente. As abordagens intensivas e em regime de ambulatório não comprometem a eficácia do tratamento e as terapias de alta pressão são eficazes e bem toleradas. O atual tratamento médico do linfedema carece de resultados a longo prazo e necessita de alternativas terapêuticas mais eficazes.
Palavras-chave: Linfedema, membros inferiores, tratamento médico
Background
Lymphedema is a progressive non-pitting edema(1) characterized by protein-rich interstitial fluid accumulation secondary to lymphatic insufficiency,(2) presenting increased tissue fluid, fibrosis, adipose hypertrophy, inflammation and pathological lymphangiogenesis.(3)
The primary form is characterized by intrinsic abnormalities in the development of lymphatic vasculature,(4) while secondary lymphedema occurs in response to an external trigging factor.(4) Primary lymphedema has been associated with mutations in several genes, including VEGFR3 (Milroy’s disease), FOXC2 (lymphedema-distichiasis) and SOX18 (hypotrichosis-lymphedema-telangiectasia syndrome). The causal gene determines disease phenotype, lymphedema mechanism and age of onset, from which it is classified in congenital, praecox (pubertal) and tarda.(5) Therefore, the specific genotype will have implications on lymphedema best approach.(6)
Lower limb lymphedema (LLL) affects around 6 million people worldwide.(7, 8) Filariasis and cancer-related lymphedema are the most prevalent forms in undeveloped countries and in the industrialized world, respectively.(4, 9)
Lower limb swelling induces symptoms of tightness, pain, heaviness, redness, restricted movement and skin thickening, which are precipitated or exacerbated by long periods of standing, walking, sitting and heat.(10) Lymphedema unique characteristics include “peau d’orange” and a positive Stemmer sign.(11) Clinical categorization is based on the International Society of Lymphology (ISL) classification(12) and Common Terminology Criteria for Adverse Events v3.0.(13)
Lymphedema complications comprise secondary infections, including lymphangitis or cellulitis(14, 15) and neoplastic processes, such as lymphangiosarcoma and angiosarcoma.(2)
Diagnostic methods include tape measurement(16), lymphoscintigraphy,(17) near infrared fluorescence imaging,(18) magnetic resonance lymphangiography(16) and bioimpedance.(19) LLL proved to negatively impact patients quality of life(20) and degrees of productivity.(7)
Lymphedema integrated treatment include non-surgical and surgical approaches, though not healing the underlying disease process. Classical physiotherapeutic techniques include drainage massages, pneumatic devices, compression bandages and hosiery.
The present literature review aims to compile current scientific knowledge on the various domains of the non-surgical treatment of LLL.
Methods
Search was performed in PubMed database, using the following medical subjects heading (MeSH) terms: “lymphedema”, “lower limbs” and “medical treatment”. Research and review articles indexed between January 2009 and December 2018 and written in English language were selected. Animal experimentation works and single case-reports were excluded. Other materials searched comprised reference books in the area of Vascular Surgery, namely Rutherford Vascular Surgery - 9th Edition, 2018.
Results
Therapy is designed regarding lymphedema stage and origin and patient functional status, ability to perform self-management and risk reduction potential.(21) Surgical approaches are mostly reserved for severe lymphedema non-responder to conservative measures.(22) Non-surgical treatment of lymphedema is divided into two main categories: primary preventive measures and interventions for those with established disease, mainly focused in reducing edema, preventing skin infection and minimizing functional loss.(2)
Target therapies have been emerging to combat the lack of efficacy of the recent approaches, aiming to reverse lymphedema fundamental pathophysiology, such as mesenchymal stem cell and vascular endothelial growth factor-C therapies.(24, 25)
Preventive Treatment and Lifestyle Interventions
Monitoring fluids ingestion and reducing salt intake are often recommended,(2) but failed to prove impact on lymphedema severity.(11) Other preventive measures include skin and nail care, weight loss, aerobic exercise, limb elevation, trauma and extreme temperatures avoidance.(2, 26) Avoidance of air travel and venipuncture and compression on the affected limb are some additional preventive measures supported by limited scientific evidence.(27)
In filariasis endemic areas, transmission is interrupted by the implementation of mass drug administration with ivermectin or diethylcarbamazine citrate (DEC) in association with albendazole.(29) Recent results point to the additional role of DEC and albendazole in reversing lymphatic pathology of both symptomatic and asymptomatic pediatric population.(30)
In developed countries, preventive measures include minimal invasive oncologic surgeries and prompt resolution of the recurrent infections events.(2) Antibiotic prophylaxis should be considered in patients who have recurrent cellulitis using phenoxymethylpenicillin or cephalexin as first choice. Alternatives for patients allergic to penicillin include erythromycin and clarithromycin.(28)
Mechanical Decongestive Lymphatic Therapy
Compression is the cornerstone of mechanical decongestive lymphatic therapy (MDCT)(31) and its effects depend on tissue compliance and edema volume.(32)
Contraindications to compression therapy include decompensated cardiac failure, deep vein thrombosis and an ankle-brachial index (ABI) of 0.5 or less.(35)
External compression modalities include drainage techniques, such as manual massages or pneumatic compression, and contention measures, namely bandaging systems and compression hosiery.(26) Treatment design should be based on individual leg shape and edema fluctuations.(35)
Adjuvant thermotherapy failed to prove synergic effect and even revealed a tendency to compromise the limb volume reductions.(36) Nevertheless, compression therapy proved to be optimized by water and vacuum effect.(37, 38)
Complex Decongestive Therapy (CDT) is recognized as the standard of lymphedema treatment(39) and is usually structured in two main phases.(2, 40) The first intensive treatment phase aims to achieve maximal decongestion and is usually guided by qualified therapists who perform manual lymphatic drainage (MLD), short-stretch bandaging, remedial exercises and dermatological care.(40) The home-care maintenance phase requires patient instruction and includes the alternating use of low-stretch elastic stocking with multi-layered bandages, skin care and continued exercises,(40) according to patient individual needs.(41)
Volume changes occur primarily within the first phase(39) and transition to the second stage occurs after limb volume stabilization.(40) Therapists-guided management proved to be more effective and only a minority of patients sustain the minimum volume achieved.(40)
The inclusion of the first phase increases patient adherence, establishes more concrete objectives, enables more appropriate management of complications(40) and quickly restores patient ability to work,(42) but failed to significantly impact long-term volume reductions.(40) Largest volume reductions were seen soon after the start of the therapy and edema reached its plateau on an average of 5.0 days, favoring the possibility of shortening treatments and potentially reducing their economic burden.(43)
No additional volume reduction was provided by the in-hospital management when compared to an outpatient therapy, with self-treatment instructions.(40) Nevertheless, cases of malignant etiology, infants, lymphorrhea and complicated lymphedema should be firstly managed by an in-patient regimen.(44)
Shortened and outpatient intensive approaches provided significant improvement in elephantiasis, proving to be an appropriate option for all LLL types.(45, 46)
Malignant lymphedema can be effectively managed by CDT without MDL, that is thought to stimulate cancer spread.(47)
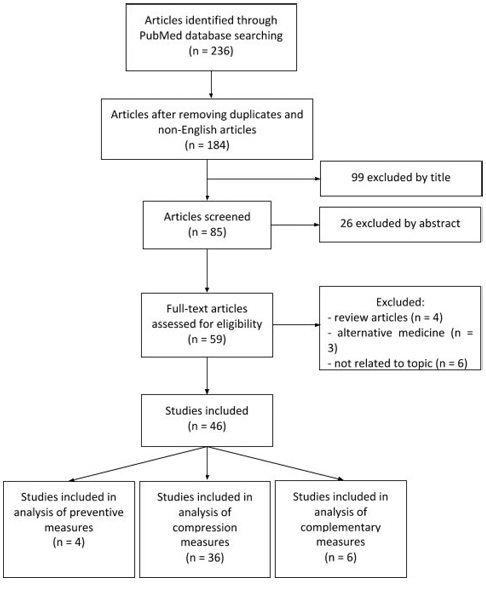
Numerous CDT treatment protocols have been proved to be an effective treatment in LLL patients, enabling significant limb volume reductions (13.8%-73.5%), with greater changes in severe lymphedema cases.(39, 42, 43, 47, 48) (Table I)
Outcome predictors of MDLT
The stone-paved ultrasonography appearance was associated with greater absolute volume reduction, while hyperechogenic subcutis predicted worse results.(46)
Subjective impact of MDLT
Compression therapy proved to significantly improve the physical and mental components of patients quality of life (QoL),(47, 49) also reducing the heaviness feeling, with positive psychological impact provided by the inclusion of exercise protocols.(50)
Leg involvement presented lower pre-CDT QoL levels, than upper extremity lymphedema patients, with higher improvements after CDT, despite similar volume reductions,(39) which was mostly due to reduction of deformity, improvement in limb function and joint motion, regression of trophic changes and the possibility of wearing a greater variety of footwear and clothing.(48)
Active treatment of malignant forms resulted in marked physical improvement and significant pain reductions.(47) Non-painful compression induced analgesia shares similar mechanisms to conditioned pain modulation with contribution of spatial summation.(51)
Manual Lymphatic Drainage
MDL applies gentle pressure to the skin, rerouting the fluid to proximal areas and softening the skin.(2) It is particularly relevant for body regions which are contraindicated to sustained pressures and should not be prescribed alone for curative purposes.(2)
Usually, MDL is preceded by manipulations of the proximal areas.(2) Drainage techniques, including pumping, scooping and rotary movements, were reported in association with tissue softening maneuvers, with 30 to 60 minutes of massage per leg.(52)
MDL therapy proved to soften the calf region, although not affecting the thigh level. It revealed a tendency to normalize tissue strains, with two opposite immediate tissue effects, increasing strain in harder tissues and decreasing strain in softer tissues, both presenting correlation between pre-MDL strains and MDL-induced changes.(52)
Pneumatic Compression Therapy
Pneumatic Compression Therapy (PCT) is performed by pump garments placed on the affected area, which alternate between inflation and deflation.(53-55)
PCT devices differ in the number of chambers, time, pressure, modes of inflation and deflation, pressure gradient and garment design(54) and are generally easy to operate on a daily-basis.(56)
Pressure and timing of PCT devices are adjustable(57) and deflation can occur either after total distal to proximal chambers inflation or sequentially after each inflation of the proximal chamber, enabling potential distal displacement of the lymph.(58)
Inflation of the pneumatic chamber proved to induce tissue compression and its partial proximal displacement, whereas deflation caused tissue decompression with recoil to a normal position.(32) Despite the increase in interstitial fluid velocity induced by chamber inflation,(59) the constant stress may result in decreased flow under its central.(59)
Efficacy of PCT depends on device parameters, but also on tissue features, since fluid accumulation and fibrosis hamper pressure transmission.(32)
The ideal pump pressure and its optimal duration is not established,(2) thus therapy design should be individualized.(11) PCT contraindications include local or proximal malignancy, limb infection, deep vein thrombosis and anticoagulation therapy.(60)
Lymph pathways during PCT
PCT proved to induce contractions of the patent lymphatic vessels and lymph movement towards the proximal limb regions, with both linear and diffuse patterns, suggesting that it occurs through the functional lymphatics and the interstitial channels.(61, 62) These subcutaneous channels develop in response to fluid accumulation and share no common feature with lymphatic endothelial cells. PCT has proven to replace their missing propelling function and to enhance their formation in proximal limb sites.(63)
Skin properties and tissue effects during PCT
Stiffness and hydraulic conductivity of tissues are crucial for determining PCT pressure distribution,(59) and skin dissipations between 40-100 mmHg were reported.(54, 57, 64)
Chamber inflation revealed limited horizontal transmission of pressure towards proximal non-compressed areas(54) with increased distal pressures during sequential inflation.(54)
The displaced tissue fluid volumes ranged from 10-40 ml per chamber, being more evident in the thigh.(54)
Similar inflation loads over the pneumatic chambers were associated with an uneven distribution of tissue fluid pressure, with lower levels in the popliteal and upper thigh regions, both containing loose connective tissue and accumulating fluid during external compression.(54, 64)
PCT usually results in decreased volume of the lower calf and thigh, with increases below the knee and in the upper thigh, evidencing fluid translocations to the popliteal fossa and to the groin region.(32, 53, 54)
Final improvement usually assumes greater expression in the calf above the ankle and mid-calf, with reported long-term maintenance.(53, 63) Maximum limb circumference reduction of 94.5% was observed in the foot area, while the thigh region revealed the greatest absolute edema reductions.(58)
Pressure and time setting in PCT devices and clinical outcomes
The level of pressure applied significantly affects PCT outcomes(8) and effective flow requires tissue fluid pressures above 30 mmHg.(59)
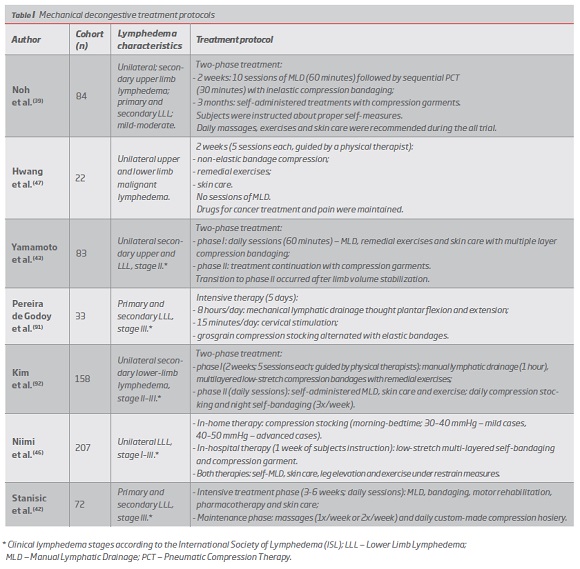
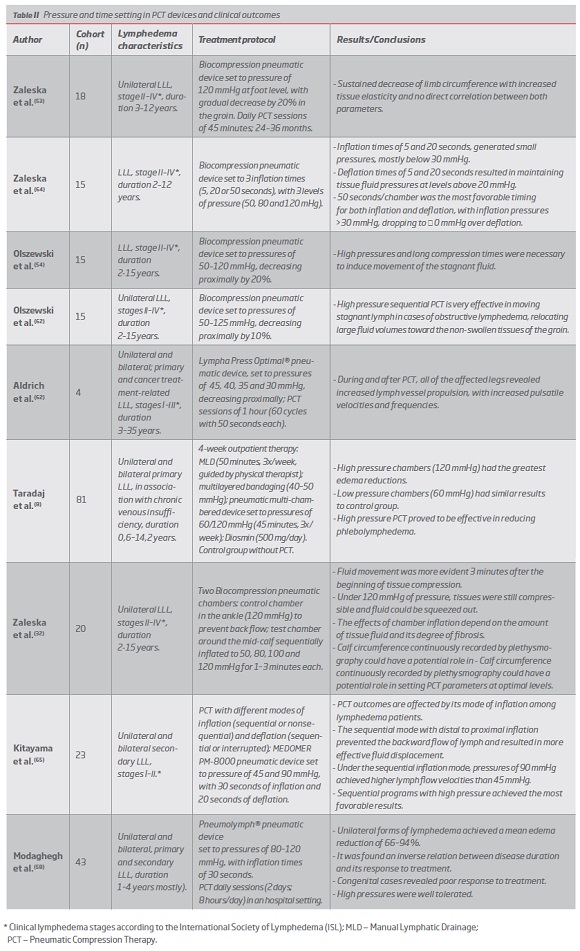
Numerous recent studies point to the efficacy of long timed (>50 seconds) and high pressure (80-120 mmHg) PCT.(8, 53, 54, 58, 59, 62, 64, 65) (Table II)
Compression Bandaging Therapy
Short-stretch bandages, with limited extensibility between 30-70%,(66) create cycles between low resting pressures and high working pressures, raising tissues hydrostatic pressure,(66) mainly in the calf.(67) Bandaging is usually combined with exercise to optimize its effects.(46)
The inclusion of low stretch bandaging before compression stocking had a significant impact on lymphedema reduction, proving to be a better restrain measure for severe forms.(46)
Bandages should apply a minimum pressure of 40 mmHg in the upright position(68) and multi-layered and high compression systems are preferable.(69)
Short-stretch compression does not accompany edema reduction and its efficacy can be compromised by non-adequate compression and slippage rates.(35, 70) Moreover, bandages discomfort and difficult application lead to poor compliance.(70)
Nevertheless, this therapy, including some self-administered techniques,(46) have revealed positive outcomes, with significant volume reductions, reduced subcutaneous fluid accumulation and improvement in pain and skin thickness.(46, 70-72)
Multi-layer bandaging have proved to be effective as an independent method among elderly lymphedema patients, with similar efficacy to CDT.(72)
Compression Garments Therapy
Medical compression garments are usually made of elasticated textile.(33) New prescriptions are required after 3-6 months (33) and skin condition should be monitored.(2)
Pressures applied vary between 20 and 60 mmHg, decreasing proximally,(73) and the highest level tolerated by the patient is considered the most beneficial.(31) However, lighter medical compression should be considered in some specific conditions, such as ABI values lower than 0.8.(35)
Pressures of 30-40 mmHg proved to better maintain treatment results than lower garments loads of 20-30 mmHg and further limb volume reduction were achieved with inelastic grosgrain stocking.(41)
Compression garments significantly impact long-term maintenance of lymphedema reduction (31, 41), improving patients satisfaction with treatment(35) and initial prescription of lower pressure stockings may be more effective.(74)
Properly fitted compression stockings add a synergistic effect to mechanical lymph drainage.(75) Thus, volume reductions of 200-300 mL make adjustment necessary.(75)
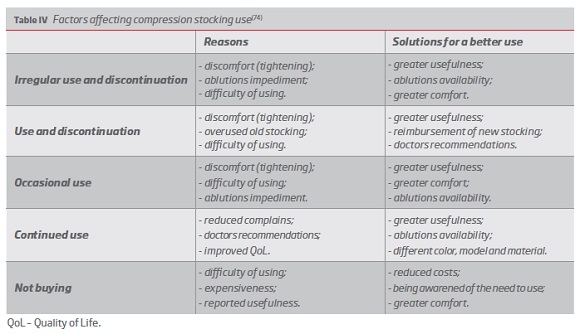
Patient incompliant use(74) (Table IV) is aggravated by potential impaired mobility.(76) The mean duration of illness was found to be 10 times higher than the average of garment use.(74) Nevertheless, treatment is accepted by the majority of patients,(74) with reported high levels of adherence to its prophylactic use.(76)
Daily compression stocking use proved to decrease the incidence of secondary lower limb swelling after lymphadenectomy, improving symptoms and clinical examination, without compromising patients QoL. However, it failed to reduce clinically significant LLL and did not affect the complication rate.(76)
Physical Exercise Therapy
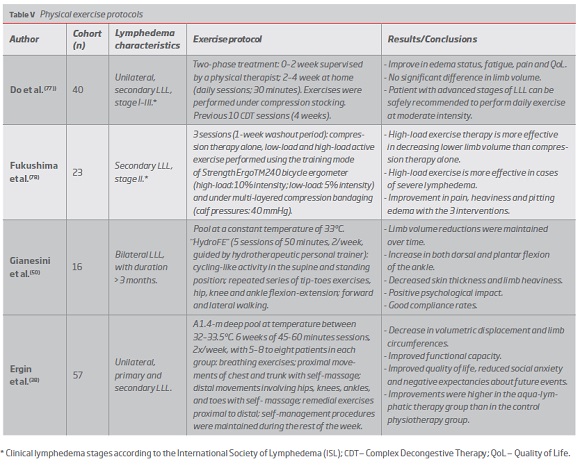
Remedial exercises and aerobic workout are part of lymphedema comprehensive approach,(2) ranging from cycling high-load exercises to aquatic protocols.(38, 50, 77, 78) (Table V)
Exercise effectiveness increases with lymphedema severity(78) and its positive outcomes include reductions in limb volume and subcutaneous skin thickness and improvements in pain, heaviness, edema status and patients QoL, mainly due to effects in fatigue and physical function. Positive psychological impact and high compliance rates are also reported.(50, 77, 78)
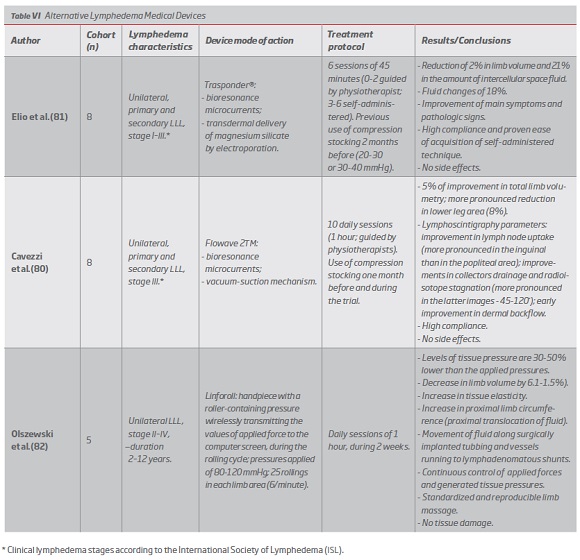
In addition to pneumatic systems, alternative medical devices have been purposed by some authors, promoting sustained volume reduction, drainage improvements and increased tissue elasticity, some even enabling its application on a daily-basis.(80-83) (Table VI)
Pharmacological Therapy
No drug therapy has been shown to be effective.(9) In patients with persistent or reluctant LLL, complementary pharmacological management with corticosteroids or diuretics(85) can be employed. Benzopyrones are not recommended due to their hepatoxicity.(86)
On histological evaluations, ketoprofen proved to significantly reverse inflammatory pathophysiological features, with no significant changes in limb volume or microlymphatic vascular area.(1)
Despite its anti-filarial action, doxycycline proved to reverse and halt the progression of inflammatory lymphedema features, suggesting its potential role in the management of non-filarial lymphedema.(87)
Intravenous pamidronate and robuvit in combination with continued conservative measures provided synergic improvements in limb volume and lymphedema-related symptoms.(88, 89)
Third-generation cephalosporins have been reported in the treatment of cellulitis with lymphangitis.(15, 90)
Discussion
Holistic approaches based on mechanical reduction of limb swelling have proved high levels of effectiveness, with reduction of lower limb volume, significant improvement on both pathological and imaging lymphedema features and subjective outcomes.(39-43, 45-48, 91) This comprehensive therapies present substantial positive impact on patients QoL, with marked improvementsin the physical components.(39, 42, 47-51) Compression therapy proved to safely manage cases of malignant lymphedema(47) and its efficacy increases with lymphedema severity.(46) It more frequently includes the combination of manual massages, bandaging, pneumatic compression, compression stocking and remedial exercises.(39, 42, 43, 46, 47, 92) In this context, CDT stands out, which is traditionally a two-phase approach with the first intensive stage usually carried out by specialized professionals. However, this first phase did not proved to significantly impact long-term results, which are mostly dependent on patient diligence.(40) Since it’s a high-cost and time-consuming therapy, shorter and outpatient versions have been promoted, with proven results,(42, 43, 91) probably reducing the economic burden without compromising treatment effectiveness.(42) Nevertheless, the majority of the studies managed the more advanced lymphedema cases in hospital-based approaches.(42, 46)
Recent studies report immediate effect of MLD on skin and subcutaneous strain. Increased tissue strains were related with collagen break and release of confined fluid, whereas the temporary removal of fluid excess was responsible for lower tissue deformity and those effects were dependent on pre-MLD tissue properties.(52)
PCT proved to be a highly effective mechanical drainage method and its benefits are largely proven.(8, 32, 53, 54, 58, 59, 61, 62, 64, 65) Despite different device parameters, most of the studies present more favorable results with long-term, high pressure (80-120 mmHg) and long inflation and deflation timed (>50 seconds) PCT with sequential modes of inflation,(8, 32, 53, 54, 58, 59, 62, 64, 65) which demonstrated to be safe and well tolerated.(53, 58, 65, 93) The obtained significant volume reductions were related to fluid translocation, in which interstitial channels play a predominant role, especially in the obstructive forms.(61, 63) For that reason, volume changes caused by PCT have been shown to vary across different levels of the lower limb, according to tissue capacity to accommodate translocated fluid.(53, 54, 58, 63) Effective tissue pressures proved to depend on pneumatic chambers applied pressures and tissues hydromechanical conditions,(59) which promoted some degree of dissipation.(54, 57, 64) Tissue effects during PCT(32, 54, 57, 59, 64) include an uneven distribution of pressures(54, 64), a decreased permeability of the compressed tissues with consequent slowing of lymph flow(59) and some backward displacement of fluid once distal positive pressures are removed.(54)
Multi-layered self-bandaging techniques have been developed(46) to create minimum discomfort in patient daily living and intelligent working dynamics, which is accomplished by the combination of elastic and inelastic components, enabling comfortable resting loads with effective pressure peaks during muscle contractions. Thin profiles are compatible with the use of clothing and footwear and minimum movement limitation.(70, 71) Recent methods have revealed significant volume reductions and imaging evidence of reduced tissue fluid, with easy and reproductible application and minimum slippage rates, as swelling goes down.(46, 70-72)
Compression bandaging systems proved to be effective as an independent method, without compromising treatment effectiveness, with the advantage of being more accessible and less labor-intensive than CDT, particularly relevant in the elderly lymphedema population.(72)
Despite the evidence of high compliance rates revealed by its prophylactic employment,(76) the major problem involving compression stocking is the considerable portion of patients that does not purchase the recommended therapy, due to inadequate use or non-application. The difficulty with its application, the costs associated and the feeling of disbelief in stocking effectiveness contribute to its incipient use. Poor compliance was associated with disease progress and increased number of hospitalizations, in a paradigm of proven high duration of illness.(74) Properly fitted compression stocking have been proved to maintain lymphedema reductions and improve patients satisfaction.(41, 49, 75, 91) Considering the importance of patient tolerance and compliance, some authors defend the prescription of lower pressures in the initial treatment phase and that garment selection process should take into account the patient’s preference.(74)
Structured exercise protocols are associated with significant reductions of chronic LLL and may be a potential first-line therapeutic approach to primary care, presenting a good cost-benefit ratio and high levels of compliance. The methods applied take into account the dependent leg position and exercises are performed in standing or lying position, frequently including bicycling activities. Studies are designed to maximize the ankle motion together with the calf and plantar muscle pumps and some authors defend the inclusion of strengthening and high-load exercises.(50, 77, 78)
Some alternative medical apparatus proved to be safe and effective, even when applied on an outpatient-basis.(45, 81, 91) Application of bioresonance microcurrents has proven beneficial effects in association with different complementary mechanisms.(80, 81) Usually, the employment of this devices is associated with high compliance rates and promotes the standardization of some therapeutic procedures.(80-82)
Recently employed pharmacological therapies include anti-inflammatory, anti-oxidant and neural mediation action.(1, 88, 89) Anti-inflammatory therapies focus on the skin as the primary target organ of the lesion, evidencing the histologic changes.(1) Some drugs have shown synergistic effects when associated with compression therapy(88, 89) and pharmacological therapy has even proved to reverse lymphedema severity.(87)
Conclusion
The non-surgical approach of LLL commits both the patient and health care providers to comply with life-long measures.
Therapeutic effects are mainly achieved by the employment of compression in the affected areas, combining external compression modalities with internal forces induced by muscular contraction.
Drainage techniques, such as manual drainage and pneumatic compression, are associated with tissue fluid translocation towards the limb areas with the lowest hydraulic resistance. Despite being associated with increased circumference in those areas, they allow marked limb volume reductions specially in the initial stages of the treatment. Moreover, decreased fluid stagnation prevents secondary tissue fibrosis and progression to more severe forms.
Contention measures, including bandaging and compression hosiery, are designed to preserve the volume reductions achieved by drainage techniques, although they may induce further limb volume reductions. Poor compliance lies in difficulties with their application and induced discomfort, associated with prolonged required time of use. Continuous adjustments are necessary, taking into account edema evolution.
The greatest advantage of physical exercise lies in the application of physiological mechanisms, which promote substantial positive effects on functional capacity and psychological state.
For this reason, patient education and motivation should be considered central aspects of non-surgical therapy. Patients involvement in treatment decisions and personalized professional care allows better transmission of the techniques and establishes more concrete goals, ultimately promoting a greater belief in therapy effectiveness.
Some systemic approaches, such as anti-inflammatory therapy, have been under recent investigation, raising hope for meaningful advances in the near future.
Limitations of the conservative treatment highlight the importance of preventive measures. Anti-filarial therapies recently proved to reverse lymphatic pathology, suggesting that they may play a role in lymphedema treatment.
Research into the molecular pathogenesis will probably allow the development of effective target therapies, that might become a reality in the future.
Health professionals lack proper education about the correct diagnostic and therapeutic procedures, thus the future of the LLL approach must be filled by increased global awareness, improved education and more effective therapeutic interventions.
REFERENCES
1. Rockson SG, Tian W, Jiang X, Kuznetsova T, Haddad F, Zampell J, et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI insight. 2018;3(20). [ Links ]
2. Sicard GA. Rutherford's Vascular Surgery and Endovascular Therapy. Journal of vascular surgery. 2018;68(5):1611-2. [ Links ]
3. Jiang X, Nicolls MR, Tian W, Rockson SG. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annual Review of Physiology. 2018;80(1):49-70. [ Links ]
4. Adamczyk LA, Gordon K, Kholova I, Meijer-Jorna LB, Telinius N, Gallagher PJ, et al. Lymph vessels: the forgotten second circulation in health and disease. Virchows Archiv : an international journal of pathology. 2016;469(1):3-17. [ Links ]
5. Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. The Journal of Clinical Investigation. 2014;124(3):915-21. [ Links ]
6. Ho B, Gordon K, Mortimer PS. A Genetic Approach to the Classification of Primary Lymphoedema and Lymphatic Malformations. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2018;56(4):465-6. [ Links ]
7. Moffatt CJ, Franks PJ, Doherty DC, Williams AF, Badger C, Jeffs E, et al. Lymphoedema: an underestimated health problem. QJM : monthly journal of the Association of Physicians. 2003;96(10):731-8. [ Links ]
8. Taradaj J, Rosinczuk J, Dymarek R, Halski T, Schneider W. Comparison of efficacy of the intermittent pneumatic compression with a high- and low-pressure application in reducing the lower limbs phlebolymphedema. Therapeutics and clinical risk management. 2015;11:1545-54. [ Links ]
9. Rockson SG, Rivera KK. Estimating the Population Burden of Lymphedema. Annals of the New York Academy of Sciences. 2008;1131(1):147-54. [ Links ]
10. Salani R, Preston MM, Hade EM, Johns J, Fowler JM, Paskett EP, et al. Swelling among women who need education about leg lymphedema: a descriptive study of lymphedema in women undergoing surgery for endometrial cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2014;24(8):1507-12. [ Links ]
11. Rockson SG. Update on the biology and treatment of lymphedema. Current treatment options in cardiovascular medicine. 2012;14(2):184-92. [ Links ]
12. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46(1):1-11. [ Links ]
13. Cheville AL, McGarvey CL, Petrek JA, Russo SA, Thiadens SR, Taylor ME. The grading of lymphedema in oncology clinical trials. Seminars in radiation oncology. 2003;13(3):214-25. [ Links ]
14. Baddour LM, Bisno AL. Non-group A beta-hemolytic streptococcal cellulitis. Association with venous and lymphatic compromise. The American journal of medicine. 1985;79(2):155-9. [ Links ]
15. Park SI, Yang EJ, Kim DK, Jeong HJ, Kim GC, Sim YJ. Prevalence and Epidemiological Factors Involved in Cellulitis in Korean Patients With Lymphedema. Annals of rehabilitation medicine. 2016;40(2):326-33. [ Links ]
16. Dixon JB, Weiler MJ. Bridging the divide between pathogenesis and detection in lymphedema. Seminars in cell & developmental biology. 2015;38:75-82. [ Links ]
17. Scarsbrook AF, Ganeshan A, Bradley KM. Pearls and pitfalls of radionuclide imaging of the lymphatic system. Part 2: evaluation of extremity lymphoedema. The British journal of radiology. 2007;80(951):219-26. [ Links ]
18. Unno N, Inuzuka K, Suzuki M, Yamamoto N, Sagara D, Nishiyama M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. Journal of vascular surgery. 2007;45(5):1016-21. [ Links ]
19. Cornish BH, Bunce IH, Ward LC, Jones LC, Thomas BJ. Bioelectrical impedance for monitoring the efficacy of lymphoedema treatment programmes. Breast cancer research and treatment. 1996;38(2):169-76. [ Links ]
20. Cemal Y, Jewell S, Albornoz CR, Pusic A, Mehrara BJ. Systematic review of quality of life and patient reported outcomes in patients with oncologic related lower extremity lymphedema. Lymphatic research and biology. 2013;11(1):14-9. [ Links ]
21. Damstra RJ, Halk A-B, Damstra RJ, Halk B, van den Berg JP, Born Y, et al. The Dutch lymphedema guidelines based on the International Classification of Functioning, Disability, and Health and the chronic care model. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2017;5(5):756-65.
22. The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology. Lymphology. 2003;36(2):84-91. [ Links ]
23. Hodgson P, Towers A, Keast DH, Kennedy A, Pritzker R, Allen J. Lymphedema in Canada: a qualitative study to help develop a clinical, research, and education strategy. Current oncology (Toronto, Ont). 2011;18(6):e260-4. [ Links ]
24. Schaverien MV, Aldrich MB. New and Emerging Treatments for Lymphedema. Seminars in plastic surgery. 2018;32(1):48-52. [ Links ]
25. Zhou Q, Guo R, Wood R, Boyce BF, Liang Q, Wang YJ, et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis and rheumatism. 2011;63(8):2318-28. [ Links ]
26. [Medical compression in the treatment of lymphedema. Haute Autorite de Sante]. Annales de dermatologie et de venereologie. 2013;140(6-7):479-82. [ Links ]
27. Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. Journal of the American College of Surgeons. 2011;213(4):543-51. [ Links ]
28. Brown L. British Lymphology Society conference 2017: Getting the basics right. British journal of community nursing. 2017;22(11):557-8. [ Links ]
29. de Kraker ME, Stolk WA, van Oortmarssen GJ, Habbema JD. Model-based analysis of trial data: microfilaria and worm-productivity loss after diethylcarbamazine-albendazole or ivermectin-albendazole combination therapy against Wuchereria bancrofti. Tropical medicine & international health : TM & IH. 2006;11(5):718-28. [ Links ]
30. Kar SK, Dwibedi B, Das BK, Agrawala BK, Ramachandran CP, Horton J. Lymphatic pathology in asymptomatic and symptomatic children with Wuchereria bancrofti infection in children from Odisha, India and its reversal with DEC and albendazole treatment. PLoS neglected tropical diseases. 2017;11(10):e0005631. [ Links ]
31. Rabe E, Partsch H, Hafner J, Lattimer C, Mosti G, Neumann M, et al. Indications for medical compression stockings in venous and lymphatic disorders: An evidence-based consensus statement. Phlebology. 2018;33(3):163-84. [ Links ]
32. Zaleska M, Olszewski WL, Durlik M, Kaczmarek M. A Novel Clinical Test for Setting Intermittent Pneumatic Compression Parameters Based on Edema Fluid Hydromechanics in the Lymphedematous Calf. Lymphatic research and biology. 2015;13(3):208-14. [ Links ]
33. Partsch H. Compression therapy: clinical and experimental evidence. Annals of vascular diseases. 2012;5(4):416-22. [ Links ]
34. Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. The Journal of physiology. 2009;587(1):165-82. [ Links ]
35. Mullins M, Bock K, Bhatia A. Extremit-Ease compression garment: A review and cases. British journal of community nursing. 2017;22(Sup12):S41-s7. [ Links ]
36. Mariana VF, de Fatima GG, Maria Pde G. The effect of mechanical lymph drainage accompanied with heat on lymphedema. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2011;16(11):1448-51. [ Links ]
37. Moortgat P, Anthonissen M, Meirte J, Van Daele U, Maertens K. The physical and physiological effects of vacuum massage on the different skin layers: a current status of the literature. Burns & trauma. 2016;4:34. [ Links ]
38. Ergin G, Karadibak D, Sener HO, Gurpinar B. Effects of Aqua-Lymphatic Therapy on Lower Extremity Lymphedema: A Randomized Controlled Study. Lymphatic research and biology. 2017;15(3):284-91. [ Links ]
39. Noh S, Hwang JH, Yoon TH, Chang HJ, Chu IH, Kim JH. Limb Differences in the Therapeutic Effects of Complex Decongestive Therapy on Edema, Quality of Life, and Satisfaction in Lymphedema Patients. Annals of rehabilitation medicine. 2015;39(3):347-59. [ Links ]
40. Suehiro K, Morikage N, Yamashita O, Okazaki Y, Hamano Md K. Impact of aggressive decongestion on the maintenance phase in combined physical therapy for lower extremity lymphedema. Annals of vascular diseases. 2011;4(4):306-12. [ Links ]
41. Pereira de Godoy JM, Pereira de Godoy HJ, Lopes Pinto R, Facio FN, Guerreiro Godoy MdF. Maintenance of the Results of Stage II Lower Limb Lymphedema Treatment after Normalization of Leg Size. International Journal of Vascular Medicine. 2017;2017:5. [ Links ]
42. Stanisic MG, Gabriel M, Pawlaczyk K. Intensive decongestive treatment restores ability to work in patients with advanced forms of primary and secondary lower extremity lymphoedema. Phlebology. 2012;27(7):347-51. [ Links ]
43. Yamamoto T, Todo Y, Kaneuchi M, Handa Y, Watanabe K, Yamamoto R. Study of edema reduction patterns during the treatment phase of complex decongestive physiotherapy for extremity lymphedema. Lymphology. 2008;41(2):80-6. [ Links ]
44. Foldi M. Treatment of lymphedema. Lymphology. 1994;27(1):1-5. [ Links ]
45. Pereira De Godoy JM, Amador Franco Brigidio P, Buzato E, Fatima Guerreiro De Godoy M. Intensive outpatient treatment of elephantiasis. International angiology : a journal of the International Union of Angiology. 2012;31(5):494-8.
46. Niimi K, Hirai M, Iwata H, Miyazaki K. Ultrasonographic findings and the clinical results of treatment for lymphedema. Annals of vascular diseases. 2014;7(4):369-75. [ Links ]
47. Hwang KH, Jeong HJ, Kim GC, Sim YJ. Clinical effectiveness of complex decongestive physiotherapy for malignant lymphedema: a pilot study. Annals of rehabilitation medicine. 2013;37(3):396-402. [ Links ]
48. Feiskhanov AK, Maksimov AV. [Comprehensive physical antiedematous therapy in treatment of patients with lymphedema]. Angiologiia i sosudistaia khirurgiia = Angiology and vascular surgery. 2016;22(4):46-50. [ Links ]
49. uchi T, Dai M, Sanada H, Okuwa M, Nakatani T, Sugama J. Associations between the treatments and outcomes of patients with upper and lower lymphoedema in Japan: a cross-sectional observational study. International journal of nursing studies. 2015;52(5):913-9. [ Links ]
50. Gianesini S, Tessari M, Bacciglieri P, Malagoni AM, Menegatti E, Occhionorelli S, et al. A specifically designed aquatic exercise protocol to reduce chronic lower limb edema. Phlebology. 2017;32(9):594-600. [ Links ]
51. Honigman L, Bar-Bachar O, Yarnitsky D, Sprecher E, Granovsky Y. Nonpainful wide-area compression inhibits experimental pain. Pain. 2016;157(9):2000-11. [ Links ]
52. Suehiro K, Kakutani H, Nakamura K, Morikage N, Yamashita O, Harada T, et al. Immediate Changes to Skin and Subcutaneous Tissue Strains Following Manual Lymph Drainage in Legs with Lymphedema. Annals of vascular diseases. 2016;9(1):30-4. [ Links ]
53. Zaleska M, Olszewski WL, Durlik M. The effectiveness of intermittent pneumatic compression in long-term therapy of lymphedema of lower limbs. Lymphatic research and biology. 2014;12(2):103-9. [ Links ]
54. Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M, Gradalski T. Tissue fluid pressure and flow during pneumatic compression in lymphedema of lower limbs. Lymphatic research and biology. 2011;9(2):77-83. [ Links ]
55. Miranda F, Jr., Perez MC, Castiglioni ML, Juliano Y, Amorim JE, Nakano LC, et al. Effect of sequential intermittent pneumatic compression on both leg lymphedema volume and on lymph transport as semi-quantitatively evaluated by lymphoscintigraphy. Lymphology. 2001;34(3):135-41.
56. Cannon S. Pneumatic compression devices for in-home management of lymphedema: two case reports. Cases journal. 2009;2:6625. [ Links ]
57. Phillips JJ, Gordon SJ. Intermittent Pneumatic Compression Dosage for Adults and Children with Lymphedema: A Systematic Review. Lymphatic research and biology. 2018. [ Links ]
58. Modaghegh MH, Soltani E. A newly designed SIPC device for management of lymphoedema. The Indian journal of surgery. 2010;72(1):32-6. [ Links ]
59. Kaczmarek M, Olszewski WL, Nowak J, Zaleska M. The Hydromechanics of Edema Fluid in Lymphedematous Lower Limb During Intermittent Pneumatic Compression. Lymphatic research and biology. 2015;13(4):260-7. [ Links ]
60. Chang CJ, Cormier JN. Lymphedema interventions: exercise, surgery, and compression devices. Seminars in oncology nursing. 2013;29(1):28-40. [ Links ]
61. Aldrich MB, Gross D, Morrow JR, Fife CE, Rasmussen JC. Effect of pneumatic compression therapy on lymph movement in lymphedema-affected extremities, as assessed by near-infrared fluorescence lymphatic imaging. Journal of innovative optical health sciences. 2017;10(2). [ Links ]
62. Olszewski WL, Cwikla J, Zaleska M, Domaszewska-Szostek A, Gradalski T, Szopinska S. Pathways of lymph and tissue fluid flow during intermittent pneumatic massage of lower limbs with obstructive lymphedema. Lymphology. 2011;44(2):54-64. [ Links ]
63. Zaleska M, Olszewski WL, Cakala M, Cwikla J, Budlewski T. Intermittent Pneumatic Compression Enhances Formation of Edema Tissue Fluid Channels in Lymphedema of Lower Limbs. Lymphatic research and biology. 2015;13(2):146-53. [ Links ]
64. Zaleska M, Olszewski WL, Jain P, Gogia S, Rekha A, Mishra S, et al. Pressures and timing of intermittent pneumatic compression devices for efficient tissue fluid and lymph flow in limbs with lymphedema. Lymphatic research and biology. 2013;11(4):227-32. [ Links ]
65. Kitayama S, Maegawa J, Matsubara S, Kobayashi S, Mikami T, Hirotomi K, et al. Real-Time Direct Evidence of the Superficial Lymphatic Drainage Effect of Intermittent Pneumatic Compression Treatment for Lower Limb Lymphedema. Lymphatic research and biology. 2017;15(1):77-86. [ Links ]
66. Farrow W. Phlebolymphedema-a common underdiagnosed and undertreated problem in the wound care clinic. The journal of the American College of Certified Wound Specialists. 2010;2(1):14-23. [ Links ]
67. Foldi E, Foldi M, Clodius L. The lymphedema chaos: a lancet. Annals of plastic surgery. 1989;22(6):505-15. [ Links ]
68. O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. The Cochrane database of systematic reviews. 2012;11:Cd000265. [ Links ]
69. Partsch H, Flour M, Smith PC. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. International angiology : a journal of the International Union of Angiology. 2008;27(3):193-219. [ Links ]
70. Schuren J, Bernatchez SF, Tucker J, Schnobrich E, Parks PJ. 3M Coban 2 Layer Compression Therapy: Intelligent Compression Dynamics to Suit Different Patient Needs. Advances in wound care. 2012;1(6):255-8. [ Links ]
71. Franks PJ, Moffatt CJ, Murray S, Reddick M, Tilley A, Schreiber A. Evaluation of the performance of a new compression system in patients with lymphoedema. International wound journal. 2013;10(2):203-9. [ Links ]
72. Zasadzka E, Trzmiel T, Kleczewska M, Pawlaczyk M. Comparison of the effectiveness of complex decongestive therapy and compression bandaging as a method of treatment of lymphedema in the elderly. Clinical interventions in aging. 2018;13:929-34. [ Links ]
73. Brennan MJ, Miller LT. Overview of treatment options and review of the current role and use of compression garments, intermittent pumps, and exercise in the management of lymphedema. Cancer. 1998;83(12 Suppl American):2821-7. [ Links ]
74. Manduz S, Ada F, Ada Y. The level of awareness and the attitude of patients recommended for use of compression stockings in Turkish society, and investigation of the factors affecting their use. Patient preference and adherence. 2018;12:399-407. [ Links ]
75. de Godoy J, #xe9, Pereira M, Lopes Pinto R, Pereira de Godoy AC, F d, et al. Synergistic Effect of Adjustments of Elastic Stockings to Maintain Reduction in Leg Volume after Mechanical Lymph Drainage. International Journal of Vascular Medicine. 2014;2014:3.
76. Sawan S, Mugnai R, Lopes Ade B, Hughes A, Edmondson RJ. Lower-limb lymphedema and vulval cancer: feasibility of prophylactic compression garments and validation of leg volume measurement. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2009;19(9):1649-54. [ Links ]
77. Do JH, Choi KH, Ahn JS, Jeon JY. Effects of a complex rehabilitation program on edema status, physical function, and quality of life in lower-limb lymphedema after gynecological cancer surgery. Gynecologic oncology. 2017;147(2):450-5. [ Links ]
78. Fukushima T, Tsuji T, Sano Y, Miyata C, Kamisako M, Hohri H, et al. Immediate effects of active exercise with compression therapy on lower-limb lymphedema. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017;25(8):2603-10. [ Links ]
79. Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta oncologica (Stockholm, Sweden). 2009;48(8):1102-10. [ Links ]
80. Cavezzi A, Paccasassi S, Elio C. Lymphedema treatment by means of an electro-medical device based on bioresonance and vacuum technology: clinical and lymphoscintigraphic assessment. International angiology : a journal of the International Union of Angiology. 2013;32(4):417-23. [ Links ]
81. Elio C, Guaitolini E, Paccasassi S, Rosati N, Cavezzi A. Application of microcurrents of bioresonance and transdermal delivery of active principles in lymphedema and lipedema of the lower limbs: a pilot study. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2014;149(6):643-7. [ Links ]
82. Olszewski WL, Zaleska M, Michelin S. A New Method for Treatment of Lymphedema of Limbs: Standardized Manual Massage with a New Device Linforoll in Conservative and Surgical Therapy Protocols. Lymphatic research and biology. 2016;14(4):226-32. [ Links ]
83. Chanwimalueang N, Ekataksin W, Piyaman P, Pattanapen G, Hanboonkunupakarn B. Twisting Tourniquet© Technique: Introducing Schnogh, a novel device and its effectiveness in treating primary and secondary lymphedema of extremities. Cancer medicine. 2015;4. [ Links ]
84. Casley-Smith JR. Benzo-pyrones in the treatment of lymphoedema. International angiology : a journal of the International Union of Angiology. 1999;18(1):31-41. [ Links ]
85. Petrek JA, Pressman PI, Smith RA. Lymphedema: current issues in research and management. CA: a cancer journal for clinicians. 2000;50(5):292-307; quiz 8-11. [ Links ]
86. Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Current medicinal chemistry. 2008;15(26):2664-79. [ Links ]
87. Mand S, Debrah AY, Klarmann U, Batsa L, Marfo-Debrekyei Y, Kwarteng A, et al. Doxycycline improves filarial lymphedema independent of active filarial infection: a randomized controlled trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(5):621-30. [ Links ]
88. Beigi AA, Sadeghi AM, Masoudpour H, Shirazinejad S, Mottaghi P. Intravenous pamidronate for refractory lymphedema. Iranian Red Crescent medical journal. 2011;13(4):263-6. [ Links ]
89. Belcaro G, Dugall M, Hu S, Ledda A, Ippolito E. French Oak Wood (Quercus robur) Extract (Robuvit) in Primary Lymphedema: A Supplement, Pilot, Registry Evaluation. The International journal of angiology : official publication of the International College of Angiology, Inc. 2015;24(1):47-54. [ Links ]
90. Micha JP, Nguyen DH, Goldstein BH. Successful management of persistent lower extremity lymphedema with suction-assisted lipectomy. Gynecol Oncol Rep. 2017;23:13-5. [ Links ]
91. de Godoy JMP, Pereira de Godoy HJ, Gracino de Marqui T, Spessoto LC, Godoy MFG. Mobilization of Fluids in the Intensive Treatment of Primary and Secondary Lymphedemas. TheScientificWorldJournal. 2018;2018:6537253. [ Links ]
92. Kim YB, Hwang JH, Kim TW, Chang HJ, Lee SG. Would complex decongestive therapy reveal long term effect and lymphoscintigraphy predict the outcome of lower-limb lymphedema related to gynecologic cancer treatment? Gynecologic oncology. 2012;127(3):638-42. [ Links ]
93. Mehrabi Bahar M, Saeed Modaghegh MH, Soltani E. Serum Lipid Changes after Short Term SIPC Therapy for Lower Limb Lymphedema. The Indian journal of surgery. 2010;72(4):305-7.
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: franciscarosas95@gmail.com (F. Rosas).
Recebido a 10 de junho de 2019
Aceite a 23 de agosto de 2019