Introduction
Post-implantation syndrome (PIS) was first described in 1999 by Velazquez et al(1) as a syndrome of fever and leukocytosis after aortic stent-graft implantation. Nowadays, PIS is defined as the clinical and biochemical expression of an inflammatory response following endovascular repair of an aortic aneurysm (EVAR).
Based on current evidence, this acute systemic inflammatory response is defined as the presence of fever (>38ºC) and leukocytosis (>12,000/¼L) according to the severe systemic inflammatory response syndrome (SIRS) criteria without any evidence of an infection.(2) This unexpected systemic inflammatory response may occur several hours or days after an EVAR. The incidence of this disease has been varying from 14 to 60%, being not systematically reported.(2,3,4)
Although this condition has been known for quite a long time, its pathogenetic mechanisms and clinical relevance are still debated. A hypothesized cause of PIS is related to the amount of new-onset thrombus within the aneurysm thrombus and its release of tumor necrosis factor-± (TNF-±), interleukin-6 (IL-6) and other cytokines within the aortic aneurysm.(4,5)
It is generally a transient benign condition with no major adverse consequences for the patients. Though, according to literature, in some patients it may negatively affect the outcome. Some severe cases have also been described, in whom serious clinical signs develop, including SIRS and multi-system organ failure with need of aggressive medical treatment and prolonged hospital stay.(6) Some studies have reported that a serious systemic inflammatory response might result in a cardiovascular or any other adverse event during the first year after EVAR or a lower quality of life during a mean follow-up of 4 years.(7,8)
Arnaoutoglou et al7 published the results of a prospective study investigating the relation of PIS with clinical outcome during the first month after EVAR. They found that the intensity of inflammation, as assessed mainly by the high-sensitivity C-reactive protein (hs-CRP) levels, correlated with the occurrence of a cardiovascular or any other adverse event.
Several laboratory biomarkers have been measured in the effort of timely predicting PIS, particularly C reactive protein (CRP), IL-6 and IL-8. Although none of these markers was found to have an adequate prognostic value.(7)
Recentently, a study has demonstrated that red blood cell distribution width (RDW) - that is a percentage resulting as the ratio between the standard deviation of mean corpuscular volume (MCV) and the value of MCV - is an independent biomarker predictor of the PIS in patients submitted to EVAR in the early postoperative period.(9)
Based on the results of the previous study, the authors investigated the population of their institution that was submitted to EVAR, to search for the incidence of PIS and an eventual relationship between PIS occurrence and various clinical and laboratory parameters.
Methods
Retrospective institutional review of consecutive patients submitted to elective EVAR (January 2015April 2020). The primary outcome was to evaluate the incidence of PIS, defined as fever (>38ºC) and leukocytosis (>12000/¼L), excluding infection through septic screening and determination of procalcitonin levels in all cases. The secondary outcomes were to identify the potential role of clinical and biomarker parameters to predict the risk of developing PIS after EVAR.
Hematological testing, which included the assessment of hemoglobin, MCV and RDW, was carried out on ethylenediaminetetraacetic acid anticoagulated blood. Delta variation of hemoglobin, MCV and RDW was calculated as the ratio between the values measured the day after the procedure and the day of admission. Operative reports were also reviewed to analyze the endovascular procedures and techniques. Categorical variables are presented as frequencies and percentages and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distribution. Normal distribution was checked using Shapiro-Wilk test or skewness and kurtosis. The evaluation of the statistical significance of the differences found was performed with Qui2 tests, Student’s t test and Fisher exact test when appropriate. All reported P values are two-tailed, with P value of 0,05 indicating statistical significance. Analyses were performed with the use of SPSS software, version 22.
The variables analyzed with statistical significance were included as predictors in a binary logistic regression model, although it was not found any association between those and the presence of PIS.
Results
According to the inclusion criteria, 107 patients were identified. Exclusion criteria were emergent settings, patients with active cancer or active infection.
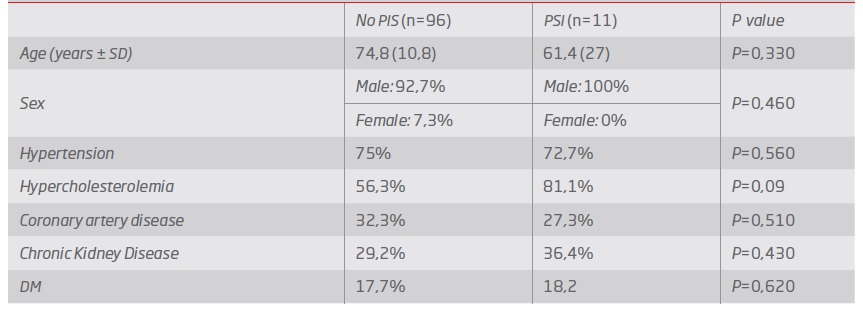
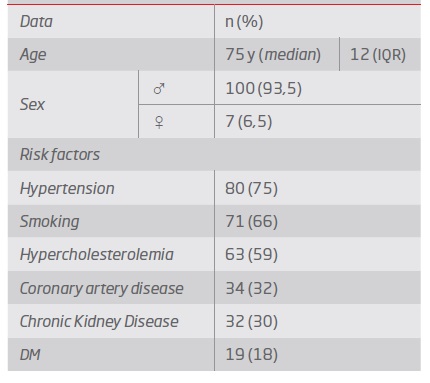
The median age was 75 years old (93.5% men). Demographic data are presented in Table 1.
Median duration of hospitalization was 5 days (IQR 2 days). The incidence of PIS was 10,2%, mostly with the peak in the first 24-48h (81% of cases). Age, sex and cardiovascular risk factors were found to be similar in both groups (P>0.05) - Table 2. Regarding the procedure approach, the majority of patients were treated with percutaneous access (72%), with no statistical influence on the occurrence of the syndrome (P=0,49).
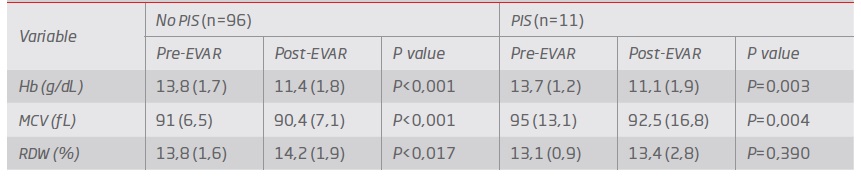
In both groups (PIS vs. no PIS), the hemoglobin values significantly decreased (P=0.04) after surgery by 14%. The same trend was observed for MCV (P=0.032), which reflected the increasing of the RDW although not reaching statistical significance - Table 3. According to the clinical process, there was no factor previously identified that could affect the value of MCV. Although delta variation of hemoglobin and delta RDW did not reach statistical significance comparing both groups (P=0,53 and P=0,07, respectively), delta MCV was found to be significantly lower in the group with PIS (P=0.012).
The stent-grafts used were Endurant II (Medtronic Inc®, Santa Rosa, CA, USA) in 80 patients and Excluder (WL Gore & Associates®, Flagstaff, AZ, USA) in 16 patients. The first is constructed with polyester and the second is constructed with polytetrafluoroethylene (ePTFE). Regarding this topic, no ePTFE graft was associated with the occurrence of PIS. The presence of PIS had significantly increased the period of hospitalization (P=0.017) from a median of 5 days (no PIS) to a median of 7 days (PIS).
Discussion
EVAR is the preferably selected treatment for AAA, although this endovascular procedure carries specific risks, including the potential development of PIS.
This study shows that PIS affects 10,2% of patients submitted to EVAR for AAA. The reported incidence of PIS in the literature varies widely from 14% to 60%.(2,3)
The etiology of PIS is not entirely clear. The endograft material is the best investigated risk factor; several studies compared the incidence of PIS or the difference in inflammatory parameters and endograft material, mainly focusing on differences between polyester and ePTFE-based structures. The majority of the studies pointed to a higher incidence of PIS or a greater increase of inflammatory markers in polyester-based endografts.(7,8,10,11,12)
In this study, no ePTFE graft was associated with the occurrence of PIS. Larger studies in this domain are needed to clarify whether the type of the endograft plays a role in the development of PIS and the justification of perioperative administration of anti-inflammatory drugs when specific types of grafts are used in order to minimize the inflammatory response.(3)
Other hypothesis is that the amount of preexisting mural thrombus within the aneurysm sac could be related to PIS development derived from the finding that mural thrombus of an aortic aneurysm contains high levels of interleucin-6.(13) According to current evidence, it is not likely that chronic mural thrombus or new-onset thrombus within the aneurysm sac play a significant role in the development of PIS. It is possible that new onset thrombus may play a small role, which could not yet be clearly demonstrated due to small sample size in all published studies on the subject.(14)
Another potential etiology for PIS after EVAR is bacterial translocation due to transient sigmoid ischemia after occlusion of a patent inferior mesenteric artery or microembolization during catheter and wire manipulations.(11) Additionally, Videm et al.(15) also suggested that contrast medium provokes neutrophil degranulation, which is greatly enhanced when combined with stent graft material, contributing to PIS occurrence.
Some demographic and clinical factors have been recognized for predicting the risk of complications after EVAR. Those include older age, female gender, renal impairment, congestive heart failure, pulmonary dysfunction and electrocardiographic evidence of ischemia.(16) Still, no single laboratory parameter has been accepted as an efficient diagnostic parameter to be routinely used for risk stratification.(16)
According to Veraldi et al8, an increase of RDW value after EVAR is a strong and independent predictor of developing PIS.
The RDW, an easy and simple parameter that can be measured by the vast majority of modern hematological analyzers, reflects the presence of anisocytosis - the variability of erythrocyte volumes, expressed in percentage, as the ratio between the standard deviation of MCV and the value of MCV. Several lines of evidence garnered over the past decade suggest that RDW is a valuable diagnostic and prognostic biomarker in patients with many cardiovascular disorders and increases upon low-grade chronic inflammation.(8,17) Elevated RDW associates with increased risk for postoperative inflammatory complications and hematopoietic tissue activity, which likely reflects a state of low-grade chronic inflammation.
According to our results, we found delta MCV to be significantly lower in the group with PIS. Delta RDW results in this study expresses that with a larger cohort it might be possible to prove the association between those parameters with the development of this syndrome.
Differences between the type of the stent graft deployed and the development of PIS might indicate that different materials and maybe configurations of the grafts, can interfere with an inflammatory response.
Some limitations should be noted in this study. The collected data was conducted in a retrospective nonrandomized fashion using a small cohort of patients. The decision for the selection of a graft type was mainly made as per the surgeons’ preference based on the expected level of technical difficulty of the procedure, and the number of patients receiving ePTFE endografts was relatively small. Moreover, we did not assess other important inflammatory markers such as interleukins and tumor necrosis factors. For this reason, the incidence of systemic inflammatory response may have been underestimated in these patients.
Additionally, the focus of this study did not include the analysis of the influence of PIS in long-term overall survival rates and other clinical outcomes.
Despite the potential limitations, our study showed the apparent benign nature of PIS after elective EVAR in acute stage, except for a longer postoperative hospital stay.
Conclusion
Abnormal hematological parameters are often observed but rarely lead to clinical consequences, as the clinical relevance of such deviations remains uncertain. The importance of having a biomarker which measurement allows the prediction of patients who have more risk to develop PIS, may help with the early management of this condition. More studies are necessary in order to stratify the risk of developing PSI in patients with AAA, especially in the early postoperative period.