Introduction
The incidence of acute renal ischemia is low. It is a rare cause of acute kidney injury, with a described incidence of 0,007% to 1,4%1. The published experience of its surgical treatment is limited to case series, and there are no well-defined indications for kidney revascularization in the presence of acute ischemia2.
Acute renal ischemia has an unspecific clinical presentation with lumbar pain and/or oligoanuria. It can be confounded with other urologic or abdominal diseases and be overlooked in clinical evaluation1. Regarding this, the diagnosis is often complicated and late1,3.
Critical considerations in management decisions (conservative or surgical) in the presence of acute renal occlusion include duration, etiology, and severity of ischemia2.
Renal arteries are considered “terminal arteries”, and renal ischemia becomes quickly irreversible if not promptly diagnosed and treated. However, the time-lapse until irreversible ischemia settles is not well defined2. Traditionally, conventional surgery was recommended, but it is associated with significant morbimortality in the presence of acute kidney injury.
This study aims to characterize the population with acute renal ischemia that undergoes revascularization in our department to predict which patients will benefit from this approach and improve this disease treatment outcome.
Methods
An observational retrospective study was performed, based on patient file analysis.
Patients who underwent renal artery revascularization in a tertiary university hospital due to acute renal ischemia, from January 2011 to June 2020, were identified. The clinical picture was characterized according to its etiology, duration, clinical manifestation, diagnosis, management, clinical, and analytic response.
The primary endpoint was dialysis rate at 30 days, defined as the percentage of living patients under dialytic therapy at 30 days postoperative.
The secondary endpoints were de novo chronic kidney disease (CKD) rate at 30 days and 30 days’ survival. De novo CKD rate at 30 days was defined as the percentage of living patients that had an estimated glomerular filtration rate (eGFR) <60mL/min/m2 according to the Cockcroft-Gault equation at 30 days postoperative (with previous eGFR ≥60mL/min/m2).
Statistical analysis was performed using IBM SPSS Statistics 24 software. Categorical variables were expressed as absolute frequencies and analyzed using the Qui-Square test or Fisher exact test. Continuous variables with normal distribution were expressed as mean ± standard deviation, and they were compared using Student’s t-test. Continuous variables with non-normal distribution were expressed as median ± interquartile range (IQR) and compared using the Mann-Whitney U test. A p-value<0,05 was considered statistically significant.
Results
Eleven patients with acute renal ischemia were included. Three were female, and the mean age was 57±14 years.
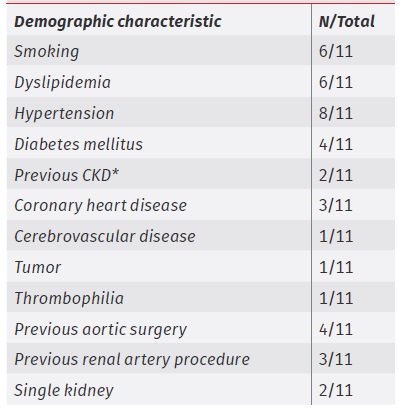
Demographic characterization is presented in table 1. Two patients had a previous CKD diagnosis, and one patient had thrombophilia (antiphospholipid syndrome plus heterozygosity for prothrombin G20210A variant). Four patients had previously undergone aortic surgery (fEVAR, TEVAR, or aortofemoral interposition graft). Three patients had previously undergone a renal artery procedure (renal stenting or aortorenal bypass due to occlusive disease, fEVAR due to aneurysmal aortic disease). Two patients had a single kidney.
The etiology of the acute renal ischemia is depicted in table 2. The most common causes of renal artery occlusion were: aortic dissection (N=3), native renal artery thrombosis (N=3, one patient with thrombophilia, one patient who previously underwent an aortofemoral interposition graft and other patient with traditional atherosclerotic risk factors), and thrombosis of previous renal revascularization (N=3, renal stent occlusion in a patient with occlusive disease, aortorenal bypass occlusion in a patient with occlusive disease, renal stent occlusion of a fEVAR).
The clinical manifestations were lumbar or abdominal pain (N=8), uncontrolled hypertension (N=5) and/or oligoanuria (N=5).
In every patient, the diagnosis was established after CTA, and the main renal artery was affected (N=9 from its ostium). In three patients, there was a bilateral renal artery occlusion. On CTA, there was some degree of contrast enhancement by affected renal parenchyma in all patients.
The median time from the first clinical manifestation to the vascular procedure was 24 hours (IQR=12-72). A patient underwent renal revascularization in less than 12 hours; six patients between 12-24 hours, and four patients in more than 24 hours.
In all patients, unilateral endovascular revascularization of a renal artery with angiographic success was performed (Fig.1), excepting one of the patients with bilateral renal ischemia, who underwent endovascular revascularization of both occluded renal arteries.
Excepting one patient with stent occlusion (that underwent drug-coated balloon or DCB angioplasty), every patient underwent stent angioplasty (N=6 using covered stents - Fig-2). Two patients previously underwent catheter-directed thrombolysis (CDT); one had embolic acute renal ischemia with 72 hours of progression and previous CKD, and underwent CDT during 24 hours; the other had thrombotic acute renal ischemia as an early postoperative complication after aortofemoral interposition graft, and underwent CDT during the minutes that preceded stent angioplasty. A patient with bilateral renal stent occlusion and a deployed fEVAR underwent percutaneous thrombus aspiration before stent deployment.
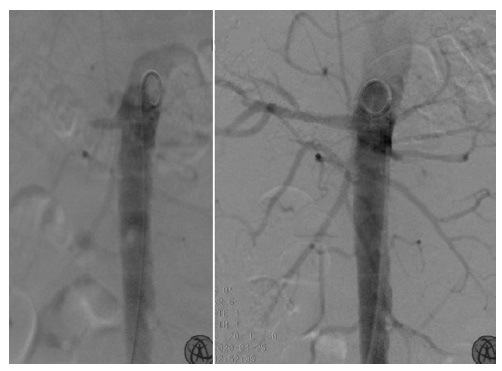
Fig.1 Endovascular revascularization of the right renal artery in a patient with traumatic bilateral acute renal ischemia. Aortic angiography (left) depicted patency of the right renal artery ostium; a right renal artery angioplasty using a covered stent was performed, with angiographic success (right).
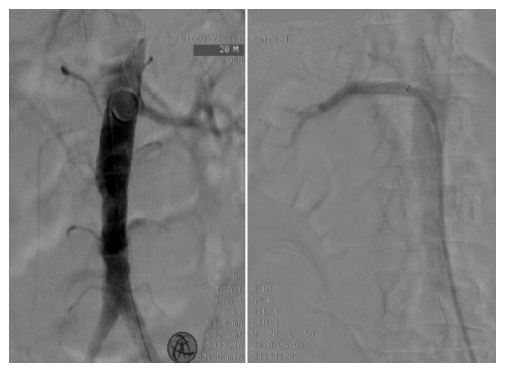
Fig.2 Endovascular revascularization of the right renal artery in a patient with thrombotic unilateral acute renal ischemia. Aortic angiography (left) depicted the left renal artery's patency without visible right renal artery; it was performed a right renal artery angioplasty using a covered stent with angiographic success (right).
The technical success was 100%. There were no procedure-related intraoperative complications.
Four patients required perioperative care in an intensive care unit (ICU), during 3, 7, 10 and 23 days, respectively.
In the postoperative period, two patients presented oligoanuria, and four underwent at least one dialysis session.
A 65 years-old male patient died on the 15th postoperative day due to multiple organ failure. He had a thrombotic unilateral acute renal ischemia with 48 hours of progression, associated with a cardiogenic shock complicating an acute myocardial infarction, and had undergone a percutaneous coronary intervention. He was hospitalized in an ICU under dialytic therapy when he died.
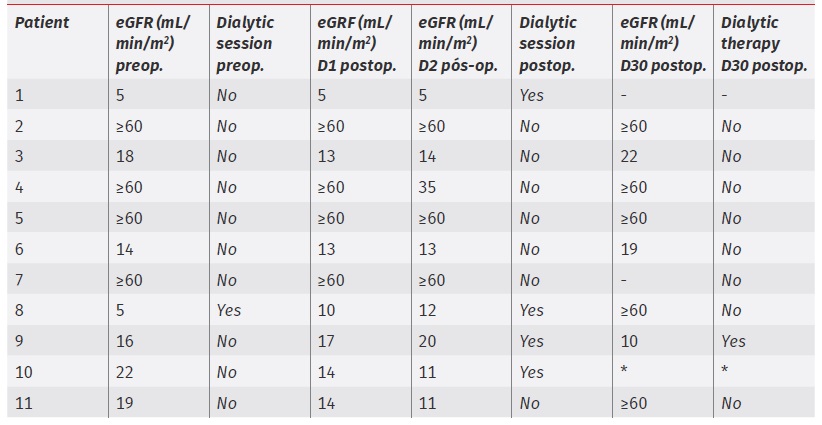
The pattern of eGRF and dialytic therapy requirement in the postoperative period is depicted in table 3.
There was no diagnosis of early occlusion of performed renal revascularization due to the clinical picture of acute renal ischemia.
It was possible to evaluate the clinical outcomes at 30 days of 9 of 11 patients. One patient died on the 15th postoperative day (above-mentioned - Patient 10), and one patient returned to his home country on the 10th postoperative day (Patient 1).
The dialysis rate at 30 days was 11% (N=1/9). He was a 48 years-old male patient (Patient 9) with traumatic bilateral acute renal ischemia (after a 12 meters fall) with 13 hours of progression, who underwent a unilateral renal angioplasty using a covered stent.
At 30 days, the rate of de novo CKD was 22% (N=2/9). Besides Patient 9 mentioned above, a 45 years-old female patient with a thrombotic acute renal ischemia with 24 hours of progression as an early complication of aortofemoral interposition graft (Patient 3) had CKD not requiring dialytic therapy at 30 days.
Thirty days’ survival was 90% (N=9/10). Patient 10 died due to multiple organ failure after complicated acute myocardial infarction.
Discussion
The most common causes of acute renal ischemia are cardiac embolism in middle and advance aged patients and trauma in young patients. Other causes include in situ thrombosis or aortic or renal dissection3-5.
It is a severe disease with unfavorable outcomes: in a study with 62 patients, 40% died or became CKD patients1.
Factors that can change the renal function recovery potential and should be considered when revascularization is a management option include previous renal function, renal collaterals and acute renal ischemia duration2,3.
A literature review concluded that only 39% of acute renal ischemia patients are diagnosed on the hospital admission day6. Some authors suggest 90 minutes as the threshold for renal function recovery; they argue that successful revascularization of longer renal artery occlusions may not precede renal function recovery, mainly if there is already renal failure or anuria2. However, in clinical practice, it is hard to achieve that time goal in diagnosing and treating this overlooked disease6. As in this study, there are several reports of renal function recovery after renal artery occlusions with more than 24 hours of progression, and other factors may be involved7. The renal parenchyma that is not irreversibly infarcted during the ischemic injury (penumbra area) may be kept healthy by late revascularization3. Therefore, some authors advocate that the renal artery occlusion duration does not predict the presence of renal parenchyma irreversible infarction6. In this way, the CTA contrast enhancement by the affected kidney can point penumbra areas that may predict recovery of previous renal function. In this study sample, the affected kidneys always showed some degree of contrast enhancement. At the same time, our study supports the idea that renal revascularization, even if late, is useful to recover renal function in many patients.
On the other hand, other case series state that it is impossible to recover renal function in the presence of main renal artery total occlusion, a condition that was present in every revascularized patient in this stydy8.
As verified in this study, some patients show admission serum creatinine within reference range; thus, a normal serum creatinine value does not exclude the diagnosis of acute renal ischemia2. In the presence of lumbar pain with or without oligoanuria, acute renal ischemia should be excluded. On the flip side, serum creatinine postoperative pattern may not reflect renal function recovery due to contralateral renal function or other factors3. Similarly, the main renal artery or renal artery branches repermeation does not predict renal function recovery, as it does not evaluate tubular function3,5. For these factors, these endpoints were not selected for this retrospective analysis.
The recommended therapeutic strategy is also controversial. Some authors recommend isolated anticoagulation, particularly if unilateral embolism in patients with limited renal recovery potential; other authors recommend open renal embolectomy, CDT or percutaneous thrombectomy3. Stent angioplasty excluding thrombus from the renal artery lumen was the most used technique on this study sample. Possible explanations include patients’ characteristics, admission’s surgical risk and institutional experience. Embolic protection devices may assist renal angioplasty procedures and could decrease the risk of embolization to segmental renal artery branches, but they were not used in the studied sample5.
This study presents some limitations recognized by the authors. It is a retrospective study performed after patient file analysis; thus, it lies in a reliable clinical registry and non-evaluable subjectivity of management decision conferred by operator experience. This study has a short sample, justified by the low incidence of both the disease and procedure in our department. It was not possible to evaluate the blood pressure pattern or which patients presented postoperative polyuria. The blood testing and imagiological follow-up after renal revascularization are not standardized in our institution. It was not possible to identify patients with acute renal ischemia who underwent conservative treatment.
Conclusion
In patients with acute renal ischemia, it is possible to revert the clinical picture and preserve renal function even after prolonged renal artery occlusions. However, it is not possible to certainly predict which patients will recover previous renal function after urgent revascularization with angiographic success.
As a quick and less invasive procedure, endovascular treatment is the first surgical option in the management of acute renal ischemia in our institution.