Introduction
Renal artery aneurysms are infrequent, occurring in about 0.1% of the population, increasing to 0.3% to 2.5% in angiographic and computed tomography studies.1,2 They are usually accidental findings on diagnostic imaging or are diagnosed in the study of secondary arterial hypertension. They can have different etiologies but are typically associated with fibromuscular dysplasia.3 The fact that fibromuscular dysplasia occurs more frequently on right-sided kidneys than on the left side could justify these aneurysms being commoner on the right side.4 Other etiologies include Behcet's disease, Von Recklinghausen's disease, arterial dissection, iatrogenic injury,
and atherosclerosis, the latter in elderly patients.5
Usually asymptomatic, renal artery aneurysms may present with lower back or flank pain and hematuria. Also, it can present with renovascular hypertension mainly due to concomitant renal artery stenosis.6
Computed tomography angiography (CTA) is usually the diagnostic method used for suspected renal artery aneurysms. However, the visualization of aneurysms in the renal artery and hilar branches may be limited. In such cases, multi-angle catheter angiography may be beneficial in facilitating the definition of the therapeutic strategy.7
There is still some controversy regarding the treatment criteria considering the growing evidence that this disorder has a benign behavior. There is some consensus that endovascular treatment may be a good option for aneurysms located in the trunk of the renal artery8. However, the evidence is not clear in cases of renal branch artery and multiple aneurysms. In these situations, ex-vivo reconstruction with cooling of the kidney offers the possibility of performing complex vascular reconstructions while preserving the functioning renal mass.
We present a complex case of bilateral renal artery branches multiple aneurysms. A detailed review of the clinical data was performed. Patients' informed consent was obtained for publication of the case report.
Case-presentation
A 35-year-old woman with a history of arterial hypertension (AHT) being treated with three different classes of drugs has been exhaustively investigated in the suspicion of secondary AHT. No laboratory abnormalities were found. The patient performed an abdominopelvic magnetic resonance angiography (MRA) to exclude renovascular hypertension, namely renal artery stenosis associated with fibromuscular dysplasia. This imaging exam revealed the presence of bilateral saccular renal artery aneurysms, measuring 24mm on the right kidney and approximately 19mm on the left kidney (figure 1), with no evidence of morphologically significant stenosis in the renal arteries. No aneurysmal dilatations were observed in other arteries of the abdomen or pelvis.
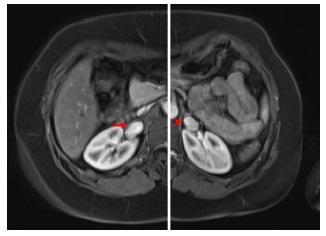
Figure 1 Abdominal Magnetic Resonance Angiography (MRA) showing a right renal artery saccular aneurysm (arrow) measuring 24mm and a left renal artery saccular aneurysm (arrowhead) of approximately 19mm in diameter with no evidence of rupture or renal artery stenosis.
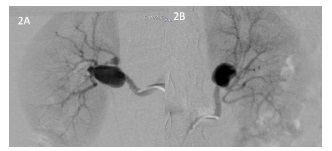
Bearing in mind that the aneurysms were located far from the ostium, apparently in a bifurcation site closer to the renal hilum, and to exclude multiple aneurysms, since bilateral aneurysms were observed, it was decided to perform a study by selective arteriography. Bilateral renal angiography confirmed the presence of saccular aneurysms of both renal arteries (figure 2).
Two saccular aneurysms were observed on the right, the largest with more than 20mm in diameter, and on the left, a saccular aneurysm with approximately 20mm. Similarly, no significant stenosis of the renal artery or its branches was observed.
The patient underwent surgical treatment with ex vivo repair and total kidney autotransplantation at different times for both kidneys, about three months apart.
The right kidney was initially treated. Transperitoneal laparoscopic nephrectomy was performed.
A brief description of the technique includes the medial mobilization of the colon to access the retroperitoneum with the opening of the renal capsule. The dissection and isolation of the ureter, artery, and renal vein is carried out. The ureter is ligated with a clip as distally as possible, and the renal vessels are ligated using two Hem-o-lok clips. The kidney is extracted into an endobag through a small Gibson incision. After rapid extraction, the kidney is washed and perfused with Celsior® preservation solution. Warm ischemia time was of 2 minutes and 25 seconds.
Upon visualization of the kidney in a bench, a large aneurysm in the renal bifurcation and another small saccular aneurysm in a first-order branch were observed. She underwent ex vivo repair of aneurysms with aneurysmectomy and aneurysmorrhaphy, and angiographic control was performed (figure 3). For this purpose, the kidney was placed on a radiolucent table under the fluoroscopic arch. The renal artery was cannulated, and approximately 10cc of a mixture of equal parts of Visipaque® 320 (Iodixanol) isosmolar, radiographic contrast medium, and Celsior® preservation solution was injected.
The on-table angiography revealed the presence of a third aneurysm in another branch, corrected with aneurysmorrhaphy. Completion angiography confirmed the permeability of all branches and adequate correction of the aneurysms. The graft was implanted in the right iliac fossa in a heterotopic fashion through the same kidney extraction incision. After dissection and isolation of retroperitoneal iliac vessels, the renal vein was anastomosed to the external iliac vein in an end-to-side fashion. It was necessary to perform an elongation venoplasty using a spiral saphenous vein graft. The renal artery was connected to the external iliac artery in an end-to-side fashion with an interposition great saphenous vein graft.
Creation of neoureterocystotomy according to Paquin's direct implantation technique was performed with insertion of a double J catheter. After seven days,
the patient was discharged with normal renal function confirmed by renal scintigraphy. The double J catheter was removed after four weeks.
After three months, an identical procedure on the left side was performed with warm kidney ischemia of
2 minutes and 59 seconds. Upon inspection, a large aneurysm was observed in the renal artery bifurcation and another small aneurysm in one of the branches; both corrected with aneurysmectomy and aneurysmorrhaphy. Ex-vivo angiographic control ensured the preservation of graft arteries patency without evidence of residual aneurysms. The kidney was uneventfully implanted in the left external iliac vessels (figure 4).
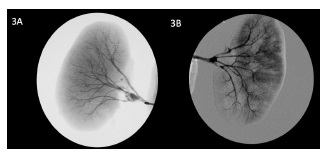
Figure 3 On the bench angiography after ex vivo repair of renal artery aneurysms before implantation on iliac vessels.
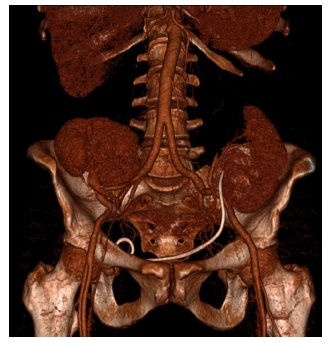
Figure 4 Postoperative Computed Tomography Angiography (CTA) reveals properly implanted kidneys in both iliac fossae. A small area of infarction is noted at the upper pole of the left kidney. A double-J catheter in the ureter of the left kidney is observed considering the short time from surgical repair.
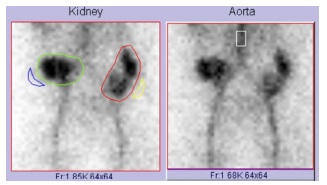
A study with renal scintigraphy confirmed the adequate function of both kidneys (figure 5). The patient was discharged after seven days without complications.
At nine months of follow-up, the patient is asymptomatic with serum creatinine values of 76.04 µmol/L (IU), corresponding to a glomerular filtration rate of 86.1 mL/min/1.73 m². Pathological examinations of the aneurysms revealed a myxoid wall degeneration, with no other specific findings. Arterial hypertension resolved utterly, and it was possible to discontinue all antihypertensive drugs with blood pressure values within the reference ranges.
Discussion
Recent studies have revealed that renal artery aneurysms usually have a more benign clinical course than previously thought, with a slow growth rate. Although there are no sufficiently robust studies, most recommendations have been increasingly broad about dimensions for treatment.9,10 Reported growth rates range from 0.65mm per year to 3mm per year, but the series with the highest number of patients points to less than 1mm per year.5,11,12
Accepted indications for treatment include aneurysms larger than 30mm in diameter or symptoaematic. Pregnancy has been associated with increased rupture rates, presumably secondary to hemodynamic changes and increased vascular volume and flow during pregnancy. Thus, the treatment of renal artery aneurysms in women of childbearing age, regardless of size, is justified, which was the case of our patient, who intended to become pregnant in the short term.
Clinically relevant renal artery stenosis is present in 7% to 66% of renal artery aneurysm patients in all series. Although renal artery occlusive disease is not evidenced in all renal artery aneurysm patients with hypertension, it remains a valid indication for intervention.(9) Our patient was one of these cases who had resistant hypertension without any evidence of stenosis or flow obstruction in the MRA and multi-angle selective angiography. The mechanism for hypertension in these patients is not fully established. However, it is thought that flow turbulence in the aneurysmal sac or eventual vascular compression causing dynamic stenosis may contribute. Aneurysmal sac thrombus embolization with cortical ischemia is another possible mechanism.5,13
Arteriography, previously considered the gold standard, was replaced by less invasive imaging tests in diagnosing and characterizing this pathology, such as CTA and MRA. These exams allow an adequate assessment of the dimensions of the aneurysms, their anatomical location, and their relationship with adjacent structures. However, selective arteriography may be essential when there is a suspicion of more complex aneurysms, namely in distal or hilar aneurysms with involvement of the renal artery branches that may be lost on cross-sectional imaging.2 Our case demonstrated this, in which aneurysms of the renal artery branches were only visualized on arteriography, which was essential in therapeutic planning.
Several therapeutic options are available, however, choosing the most appropriate treatment remains controversial. With the increasing use of endovascular techniques, simple and proximal renal artery trunk aneurysms could be treated by placing a stent-graft, selective coil embolization or balloon-assisted coil embolization with reported success rates of 73% to 100% and a morbidity rate of 13% to 60%.2,14,15
Postembolization syndrome, defined as a combination of fever, leukocytosis, abdominal pain, nausea, and vomiting, was the most common reported complication.16
Endovascular intervention in more complex renal artery aneurysms results in high incidences of the post-embolization syndrome and segmental renal ischemia. Also, there are uncertain long-term data.5
In more complex aneurysms, surgical repair is the treatment of choice. Several techniques are described and include in-situ repair, ex-vivo reconstruction with partial (without division of the ureter) or total kidney autotransplantation, and primary nephrectomy.
In situ repair can be performed by conventional open, laparoscopic, or robotic approach.17 Usually used for single aneurysm repair, in situ repair is not recommended in complex aneurysms of bifurcations or branches or multiple aneurysms when a long ischemia time is expected to perform the vascular reconstruction.18 Available conventional in situ reconstructions include aneurysm resection with primary angioplasty (with or without branch reimplantation), patch angioplasty, primary anastomosis, interposition bypass, aortorenal bypass, splanchnic-renal bypass, and small aneurysm plication.
Although historically treated with nephrectomy, current data support that complex distal branches, or multiple aneurysms are best addressed with ex-vivo repair and renal autotransplantation, providing durable repair and maximum graft protection.
This technique somehow increases with less invasive procedures for kidney harvest. Laparoscopic living donor nephrectomy reduced hospital stay, pain, and time to return to full function.19 However, availability and experience should be the decisive factors.
At our center, experienced laparoscopic urologists are integrated into the surgical teams to perform autotransplant procedures, so we use the laparoscopic technique whenever possible.
Although direct studies comparing in situ reconstructions and ex-vivo repairs have not been performed, better patency and renal viability rates have been observed in ex-vivo reconstructions.8,20
Murray et al. describe the most extensive series with a 92% success rate with 12 patients treated without significant mortality or morbidity.21 Gallagher et al. reported seven ex-vivo reconstructions after laparoscopic nephrectomy for complex aneurysmal disease to avoid surgical incision morbidity; these authors described excellent technical success, no mortality,
no ureteral morbidity, and a 28% incidence of perioperative morbidity.22 Duprey et al. showed primary patency at 30 days of 90.8% with a survival of 95% at nine years.5
Data from our center with the kidney autotransplantation technique were recently published with a kidney patency rate of 93% with a follow-up of 47.2 months and no mortality.12 As in this case, we use laparoscopic surgery to harvest the kidney in most cases due to the excellent expertise of the surgical team, decreasing surgical aggressiveness and morbidity.
Primary nephrectomy is currently a treatment only used in extreme cases of multiple complex aneurysms, refractory hypertension, or when a reconstructive surgical solution cannot be performed, namely after the failure of endovascular treatment.18 In case of a ruptured renal artery aneurysm with hemodynamic instability, it may be an option. However, all efforts must be made to preserve the functioning renal parenchyma.
Conclusion
In our experience, renal autotransplantation with ex vivo arterial repair seems to be a good solution in treating multiple and complex renal artery aneurysms, with good mid and long-term results. The results in the literature corroborate this evidence with a high success rate, low morbidity and mortality, high patency rates and preservation of renal function.