Introduction
Shoulder dislocation is a very common shoulder injury that may result from a fall, sports or trauma. Multiple complications have been reported, most commonly recurrent dislocation, axillary nerve injury and bone defects. Axillary artery injury secondary to a shoulder dislocation without bone fracture is extremely rare. Less than 1% of axillary artery injuries are caused by this type of mechanism(1). The high energy associated with these accidents may cause a great displacement of both the humeral head and axillary artery, causing rupture of the artery or severe lesions of the inner layer and adventitia, which may trigger its occlusion 2. Brachial plexus injury is relatively less common than that of isolated axillary nerve.
Concomitant orthopedic and vascular injuries are associated with a high rate of limb loss with reports ranging from 18% to 38% 3.
The best intervention to restore blood flow or to stop hemorrhage is not always clear. Most cases of extremity vascular trauma have been treated with open revascularization procedures.
Minimally invasive percutaneous endovascular therapies offer an attractive treatment alternative.
Case report
A 74-year-old man presented to the emergency department 1 hour after a fall at his home. He suffered a forced abduction injury of his right shoulder in an attempt to avoid falling. The patient was conscious and cooperative. He had no respiratory distress and was hemodynamically stable. His medical record included an episode of anterior right shoulder dislocation, mitral valvuloplasty, bilateral total hip replacement and dyslipidemia. He was medicated with Warfarin, furosemide 40 mg, twice a day, and a statin.
The physical examination suggested a right shoulder dislocation because of the extra-anatomical shoulder position. He was unable to move his arm. He had a cold, pulseless and painful hand with loss of sensorimotor function. Hand-held doppler found no audible signals in the right arm.
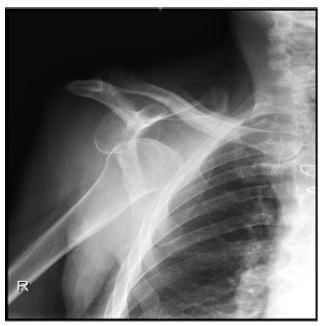
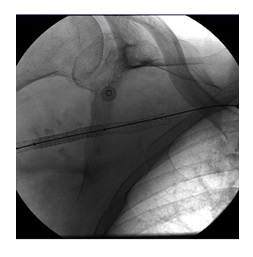
A shoulder X-ray confirmed an anterior shoulder dislocation without fractures (Fig.1).
The shoulder was repositioned in the emergency department, Kocher technique was used for the reduction.
After repositioning, a vascular assessment confirmed the presence of a vascular injury. From a neurological standpoint he appeared to have a severe brachial plexus palsy.
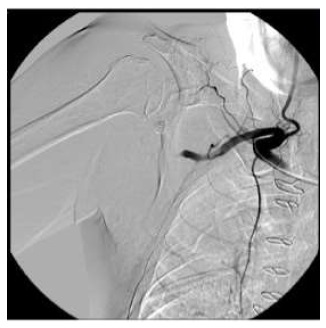
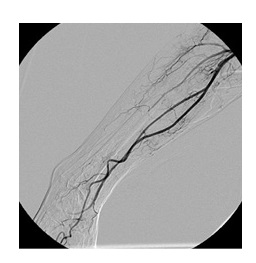
Angiography via a right femoral arterial puncture was performed. For added support we used a 7 Fr introducer with 70cm (Flexor, Cook). A selective catheterization of the right subclavian artery showed an abrupt occlusion of the axillary artery without contrast extravasation (Fig. 2). Since the patient was anticoagulated (and taking into account the risk of bleeding) an endovascular approach was chosen as alternative to open surgery.
The patient had a pain that was difficult to control and despite having an INR of 3,4 we chose not to wait for the warfarin reversal
We crossed the arterial injury with a hydrophilic guidewire 0.035 with the support of a 4Fr straight catheter. A 7 mm × 100 mm self-expanding stent (Zilver Flex, COOK) was placed in the location where the artery was occluded.
Considering the cause of the lesion and the absence of contrast extravasation on angiography we raise the hypothesis that we were facing an occlusion secondary to an intimal lesion. So we opted for a bare metal stent that turned out to be the wrong choice.
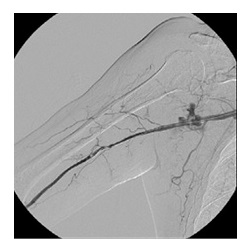
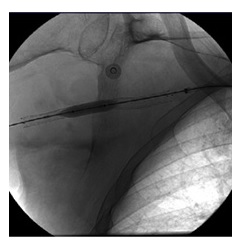
A control angiogram showed contrast extravasation at the site where the stent was placed and patency of the distal arteries (Fig.3). In an attempt to control the bleeding a 7 mm × 60 mm angioplasty balloon (Armada 35, Abbott) was inflated for a long time, unsuccessfully (Fig.4).
A 7 mm × 38 mm balloon expandable covered stent (Atrium V12, Maquet) was then placed successfully controlling the bleeding and maintaining the patency of the artery.(Fig.5 and Fig.6).
Although these stents were not the ideal choice for this location because of the risk of fracture and thrombosis, they were the only covered stents available to control de bleeding.
After the procedure the radial and ulnar pulses were palpable and hand temperature increased.
A needle electromyography was subsequently performed, showing severe brachial plexopathy.
Nerve damage was treated conservatively using a brace because of the dropping hand.
He started a physiotherapy program that will continue after hospital discharge. To date, no neurological improvement was observed.
Clinical and imagiological (with duplex ultrasound) Follow up will be performed in vascular surgery consultation every 3 months during the first year, and then every 6 months.
Discussion
Axillary artery rupture after a shoulder dislocation is very unusual. Vascular injury occurs more commonly in older patients, because of the loss of arterial elasticity secondary to atherosclerosis. The likelihood of such an event increases in cases of recurrent dislocation. Recurrent episodes of dislocation may cause adhesions between the vessel and joint capsule, again making the artery more prone to damage from shearing forces4.
Approximately 90% of the lesions occur in the third part of the axillary artery (defined as that portion distal to the lower edge of the pectoralis minor muscle). Several theories have been postulated to explain the propensity for vascular injury to occur in the third portion. Milton proposed that the artery is fixed in that location by the anterior and posterior circumflex arteries, as well as by the subscapular artery. The artery is then exposed to direct injury by the hyper abducted humeral head5. The nature of the injury might range from complete transection of the axillary to intimal tears, branch avulsion, or pseudoaneurysm formation.
Association with a severe brachial plexus lesion is common in such injuries. The presence of an ischemic limb makes preoperative clinical neurological assessment difficult6. Involvement of the brachial plexus represents the most important determinant of long-term disability.
Severe brachial plexus injury results in devastating functional disability and persistent causalgia, which may lead to repeated hospitalization and even subsequent elective amputation, regardless of patent arterial repair7.
In this case the option for endovascular treatment was due to changes in hemostasis caused by anticoagulant therapy and because the risk of adverse events in an open surgical revascularization were considerable.
The major advantage of this approach is the avoidance of open axillary artery exposure and the associated risk of injury of adjacent structures, such as the axillary vein or the brachial plexus, which in this case had a severe injury. Other advantages are the possibility of performing the procedure under local anesthesia, and the shorter hospital stay.
In this case the main disadvantage of the endovascular approach is the risk of stent fracture due to its location in the axillary region.
The durability of such procedures is unknown and requires long-term follow up.
Conclusion
Axillary artery injury after a shoulder dislocation is a rare condition. The classic treatment is open arterial reconstruction with patch angioplasty or a bypass. However, in this case we show that it can be possible to perform a minimally invasive repair of the axillary artery injury with a stent implant, avoiding the aggression and risks of open surgery, with successful outcomes.