On behalf of the Principal Investigators of the Portuguese National Registry of Vascular Procedures
Anita Quintase, Carolina Vazg, Daniel Brandãoh, Diogo Silveiraa, Emanuel Silvac, Gabriel Anacletoi, Gonçalo Cabralj, Gonçalo Queiroz de Sousak, Hugo Rodriguesl, Hugo Valentimm, Joao Vieiran, Joel Sousao, José Carlos Vidoedop, Luís Machadoq, Nelson Oliveirar, Rita Augustos, Rita Ferreirat, Pedro Sousau, Tiago Ferreirav
g Hospital de Santo António, Centro Hospitalar Universitário do Porto; Porto, Portugal; h Hospital da CUF Porto; Porto, Portugal; i Centro Hospitalar e Universitário de Coimbra; Coimbra, Portugal; j Hospital Beatriz Ângelo; Loures, Portugal; k Hospital Garcia de Orta; Almada, Portugal; l Hospital Militar Principal; Lisboa, Portugal; m Hospital da CUF Descobertas; Lisboa, Portugal; n Hospital Lusíadas Lisboa; Lisboa, Portugal; o Centro Hospitalar Universitário de São João; p Centro Hospitalar de Tâmega e Sousa; Penafiel, Portugal; q Centro Hospitalar de Trás-os-Montes e Alto Douro; Vila Real, Portugal; r Hospital do Divino Espírito Santo; Ponta Delgada, Portugal; s Centro Hospitalar de Braga; Braga, Portugal; t Hospital do SAMS; Lisboa, Portugal; u Hospital da Senhora da Oliveira; Guimarães, Portugal; v Centro Hospitalar de Lisboa Ocidental - Hospital de Egas Moniz; Lisboa, Portugal.
Introduction
The importance of monitoring outcomes following abdominal aortic aneurysm (AAA) repair has been emphasized by the European Society for Vascular Surgery (ESVS) AAA guidelines, which recommend that vascular units performing aortic surgery should record cases into a validated prospective registry to allow for monitoring of changes in practice and outcomes.1)
Registries offer data for large-scale outcome analysis, over long periods of time and in different cultural and economic settings, enabling continuous evaluation and improvement of AAA management. They also provide an objective assessment of real-world performance, evaluating the applicability of randomized controlled trial (RCT) findings in daily practice and compliance with the guidelines. Ultimately, they help decreasing the gap between evidence and practice.2,3
The oldest AAA registries in Europe are the Swedvasc Registry and the Hospital Episode Statistics (HES) in England. These initiatives allowed us to follow the evolution in the treatment of AAA, namely the introduction of endovascular aneurysm repair (EVAR) and its impact on results. Data from Swedvasc are analyzed and summarized in a yearly report, supplying the vascular community with center-specific data for continuous quality improvement projects.2
Some registries might be biased by voluntary data contributions. There are some compulsory registries, like the Dutch Surgical Aneurysm Audit (DSAA).3)
On top of reports resulting from different national or regional registries, the Vascunet Collaboration and The International Consortium of Vascular Registries (ICVR) perform international benchmarking studies of vascular surgical practice and outcome, identifying important variations in the practice of vascular surgery between countries and regions.4
The Portuguese National Registry of Vascular Procedures (RNPV) was created and financed by the Portuguese Society of Angiology and Vascular Surgery (SPACV) in 2019, according to national legislation. The RNPV is organized into modules, depending on pathology. The AAA module was the first to be introduced in 2019, followed by the carotid pathology module in 2022. Due to a built-in live statistical tool, each participating center has the opportunity to analyze their own results in real time and compare them with the national average results on several relevant outcomes.
In 2021, the first paper using data from one year of the Portuguese RNPV was published, reflecting the initial experience with this module.5
The aim of this study was to evaluate the results of the first 1000 infra-renal aneurysms included in the Portuguese RNPV focusing on characterizing the baseline demographics, modalities of repair and early outcomes.
Methods
The RNPV is a prospective, voluntary, population-based registry, accessible to all portuguese public and private departments of Angiology and Vascular Surgery. Although it does not cover all vascular centers, a very large proportion (more than 90%) participated, including all teaching and tertiary institutions in the country. Each participating center acquired local ethics committee approval. Patients' informed consent had to be obtained and willing patients signed a Consent Form designed according to the Declaration of Helsinki. All registered data are anonymized, in accordance with current legislation on data protection.
The AAA module comprises patients submitted to aneurysm repair (either elective or urgent) that involves the infra-diaphragmatic aorta, with or without involvement of the iliac arteries. Inclusion of isolated common iliac artery (CIA) aneurysms is permissible, if the disease involves or the repair includes the abdominal aorta.
Patient- and hospital-level factors were retrospectively extracted for all patients that underwent elective and urgent AAA repair, between November 2019 and December 2022. Exclusion criteria were defined as follows: (1) type 4 thoracoabdominal aortic aneurysms, which were allowed in the registry if the supradiaphragmatic aorta was not involved; (2) pararenal aneurysms; (3) juxtarenal aneurysms; (4) complex EVAR interventions, including renal and visceral branches/fenestrations/chimneys.
After applying the exclusion criteria, the first 1000 patients comprised in our database were included.
Collected data were analyzed including demographic information, treatment indication, aneurysm anatomic characteristics, type of intervention, outcome data at 30-days, 1-year and center caseload data. Center caseload per year was calculated for both elective and urgent procedures resorting to the three complete years included in the registry (2020, 2021 and 2022).
The variables “ischemic cardiac disease”, “pulmonary disease”, “cerebrovascular disease” and “renal impairment” were dichotomised per patient into categories “present” or “absent”. The first three were based on ICD-10 codes. Pre-operative renal impairment was defined as a baseline level of creatinine above 1.5 mg/dL.
The types of intervention (EVAR and open surgical repair - OSR) were compared within elective and urgent settings. The primary outcome was defined as 30-day mortality. The following secondary outcomes were also analyzed: perioperative (30-day) myocardial infarction, cerebrovascular events, pulmonary, renal and bowel complications, hospital length, reintervention rate and 1-year survival.
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations.
Statistical analysis was performed to determine the association between relevant risk factors and mortality with Fisher's exact or Pearson X2 test for categorical variables and independent sample T-Test or Mann-Whitney U Test (when appropriate) for continuous variables. Multivariable logistic regression was performed for 30-day mortality prediction analysis to adjust for possible confounders. Variables were included using a forward stepwise approach if a p value <0.05 was found in univariable analysis.
All reported p values are two-tailed, with a p value of <0.05 indicating statistical significance. Analyses were performed with the use of SPSS, version 27.0.
Results
Between November 2019 and December 2022, a total of 1122 patients were included in the AAA module of the Portuguese RNPV. During this period, a mean of 28.57 procedures per month were registered on the platform (Figure 1), with a uniform distribution over time (p=0.077).
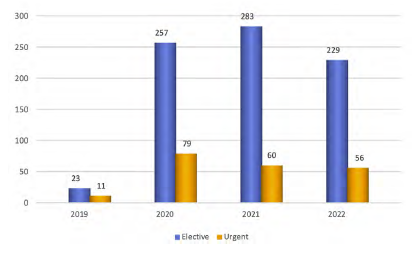
Figure 1 Number of elective and urgent procedures registered on the Portuguese RNPV platform per year
After applying the predetermined exclusion criteria, we analyzed the first consecutive 1000 patients with infra-renal aneurysm submitted to EVAR or OSR, in elective or urgent settings. Sixty-eight (6.8%) of these presented with isolated CIA aneurysms, in which abdominal aortic repair was also performed.
Baseline characteristics
Patients were predominantly male (91.8%), with a mean age of 74.1 (± 10.6) years. Patient characteristics are presented in Table 1. Admission mode and treatment indication are presented in Table 2.
Table 1 Characteristics of the first 1000 patients with infra-renal aneurysm submitted to either elective or urgent repair, included in the AAA module of the Portuguese National Vascular Registry
| Patient characteristics | |
|---|---|
| Age (mean years ± SD) | 74.1 ± 10.6 |
| Sex | |
| Male - n (%) | 918 (91.8) |
| Female - n (%) | 82 (8.2) |
| AAA diameter (mean mm ± SD)* | 66.4 ± 16.9 |
| Comorbidities | |
| Diabetes - n (%) | 223 (22.3) |
| Ischemic cardiac disease - n (%) | 332 (33.2) |
| Renal impairment - n (%) | 167 (16.7) |
| Pulmonary disease - n (%) | 217 (21.7) |
| Cerebrovascular disease - n (%) | 105 (10.5) |
| ASA classification (I-IV) - n (%) | |
| I | 3 (0.3) |
| II | 142 (14.2) |
| III | 624 (62.4) |
| IV | 228 (22.8) |
ASA: American Society of Anesthesiologists; SD: Standard Deviation. *Iliac aneurysms excluded
Table 2 Mode of admission and treatment indication of the first 1000 patients with infra-renal aneurysm, included in the AAA module of the Portuguese National Vascular Registry
| Mode of admission | n (%) |
|---|---|
| Elective | 790 (79.0) |
| Urgent | 205 (20.5) |
| Missing | 5 (0.5) |
| Treatment Indication | n (%) |
| Aortic diameter | 605 (60.5) |
| Iliac diameter | 110 (11) |
| Symptomatic aneurysm (no evidence of rupture) | 60 (6) |
| Aortic aneurysm rupture | 137 (13.7) |
| Iliac aneurysm rupture | 13 (1.3) |
| Missing indication | 75 (7.5) |
Aortic diameter was the main indication for treatment. Considering only patients treated for AAA with reported information about aortic diameter and excluding surgical indications due to iliac aneurysms (n=872), we identified 142 (16.3%) patients whom underwent EVAR or OSR for small AAA, under the diameter criteria per sex underlined by the ESVS AAA guidelines1 (Figure 2). Criteria for treatment in these patients were described as follows: aortic diameter n= 54 (38.0%); symptomatic AAA without rupture n= 15 (10.5%); aortic rupture n=8 (5.6%); and no indication identified n=65 (45.8%). No descriptional data was available.
AAA elective repair
Elective surgery was performed on 790 patients. EVAR was the preferred technique in 75.4% of these patients, which were significantly older and with more comorbidities (Table 3).
Table 3 Demographics of elective patients submitted to infrarenal aneurysm repair, included in the AAA module of the Portuguese National Vascular Registry, compared by type of surgery
| ELECTIVE SURGERY | EVAR | OSR | p value |
|---|---|---|---|
| n (%) | 596 (75.4) | 194 (24.6) | p < 0.001 |
| Age (mean years ± SD) | 75.51 ± 11.1 | 68.38 ± 7.7 | p < 0.001 |
| Male - n (%) | 550 (93.1) | 184 (95.8) | p = 0.168 |
| Aneurysm diameter (mean mm ± SD) | 57.5 ± 14.3 | 62.7 ± 16.9 | p < 0.01 |
| Diabetes - n (%) | 156 (26.2) | 34 (17.5) | p = 0.014 |
| Ischemic cardiac disease - n (%) | 222 (37.5) | 51 (26.4) | p = 0.005 |
| Renal impairment - n (%) | 84 (14.5) | 16 (8.6) | p = 0.038 |
| Pulmonary disease - n (%) | 157 (26.4) | 27 (1.0) | p <0.001 |
| Cerebrovascular disease - n (%) | 62 (10.0) | 20 (10.3) | p = 0.959 |
SD: Standard Deviation.
Table 4 Outcomes of elective patients submitted to infrarenal aneurysm repair, included in the AAA module of the Portuguese National Vascular Registry, compared by type of surgery
| ELECTIVE SURGERY | EVAR | OSR | p value |
|---|---|---|---|
| Outcomes at 30 days | |||
| Myocardial infarction - n (%) | 8 (1.8) | 10 (4.3) | p = 0.051 |
| Cerebrovascular event - n (%) | 0 | 0 | |
| Pulmonary complication - n (%) | 3 (0.5) | 17 (9.1) | p < 0.001 |
| Renal impairment requiring hemodiafiltration - n (%) | 5 (0.9) | 8 (4.2) | p = 0.002 |
| Bowel ischemia - n (%) | 2 (0.3) | 9 (4.8) | p < 0.001 |
| Abdominal compartment syndrome - n (%) | 0 | 4 (2.1) | p < 0.01 |
| Hospital length (mean days ± SD) | 5.06 ± 9.43 | 12.6 ± 12.1 | p < 0.01 |
| 30-day reintervention - n (%) | 17 (3.0) | 22 (11.8) | p < 0.01 |
| Intra-hospital mortality - n (%) | 7 (1.2) | 10 (5.3) | p = 0.001 |
| 30-day mortality - n (%) | 10 (1.8) | 11 (5.9) | p = 0.003 |
| Outcomes at 1 year | |||
| Survival status- n (%) | 156/176 (88.6) | 50/61 (82.0) | p = 0.183 |
SD: Standard Deviation.
The overall 30-day mortality in elective surgery was 2.7% (EVAR 1.8% versus OSR 5.9%; p=0.003).
All the post-operative complications were more frequent in the OSR group, with significant repercussion in hospital length-of-stay, reinterventions and early mortality (Table 4). In the study population, only the baseline AAA diameter was a predictor of 30-day mortality: mean AAA diameter of patients alive was 58.7 ± 15.0 mm, while the patients who died had a mean AAA diameter of 68.1 ± 19.6mm (p=0.007). No other pre-operative factors were predictive of 30-day mortality.
Concerning sex, we found women to be older than men at the time of the intervention (79.83 ± 30 versus 73.41 ± 8, p<0.001), with a trend towards higher intra-hospital mortality (6.4% versus 1.9%, p=0.05) and no difference in 30-day mortality (6.4% versus 2.5%; p=0.108).
The one-year survival status was not influenced by AAA repair technique. However, it is important to emphasize that follow-up data at one-year was scarce (30% of patients), mainly due to underreporting.
EVAR repair
In our study population, 706 patients were submitted to EVAR repair: 596 (84.4%) elective and 110 (15.6%) urgent procedures.
Mean aneurysm diameter, neck features and stent graft configurations are summarized in Tables 5 and 6.
Table 5 Aneurysm features of patients submitted to elective or urgent EVAR repair, included in the AAA module of the Portuguese National Vascular Registry
| Elective EVAR | Urgent EVAR | p value | |
| Aneurysm diameter (mean mm ± SD) | 57.5 ± 14.3 | 70.5 ± 21.5 | p < 0.001 |
| Neck diameter (mean mm ± SD) | 23.2 ± 3.3 | 24.3 ± 4.2 | p < 0.016 |
| Neck length (mean mm ± SD) | 29.6 ± 14.7 | 23.9 ± 12.7 | p = 0.001 |
| Median proximal oversize (mean % ± SD) | 17.6 ± 7.1 | 19.9 ± 8.8 | p < 0.008 |
SD: Standard Deviation.
Table 6 Stentgraft configuration of patients submitted to elective or urgent EVAR repair, included in the AAA module of the Portuguese National Vascular Registry
| Bifurcated device | Aortic tubular device | Aorto-uni-iliac device | ||
|---|---|---|---|---|
| Elective EVAR - n (%) | 532 (93.8) | 14 (2.5) | 21 (3.7) | p < 0.001 |
| Urgent EVAR - n (%) | 59 (56.7%) | 6 (5.7%) | 39 (37.5%) |
SD: Standard Deviation.
Percutaneous access was the most used in elective procedures (59.8%), while in the urgent setting surgical access was the preferred (54.1%).
AAA urgent repair
Urgent repair was performed on 205 patients, presenting with: AAA rupture n=137 (66.8%); iliac aneurysm rupture n=13 (6.3%); and symptomatic infrarenal aneurysm without rupture n=55 (26.8%). In the subgroup of patients with an AAA rupture, we identified 8 (5.8%) male patients with an aneurysm diameter below the diameter-threshold for AAA repair (49.8 ± 5.1mm).
The preponderance of EVAR was less pronounced comparing to OSR (53.7% versus 46.3%). Demographic features and comorbidities were similar in both groups, except for age and aneurysm diameter (Table 7).
Overall, 30-day mortality rate was 34%. Survival was better for patients treated by EVAR (28.8% versus 44.4% with OSR, p=0.024). A sub-analysis of the patients presenting with ruptured AAA identified a 30-day mortality of 33.3% and 57.3% in EVAR and OSR groups, respectively (p=0.006). Mortality of patients with ruptured iliac aneurysm was not significantly influenced by the surgical technique (EVAR 40% versus 42.3% OSR, p=0.921).
Overall, post-operative complications were not significantly different between EVAR and OSR groups, although there was a greater need for transfusion support in patients submitted to OSR (p<0.001). Bowel ischemia was more common after OSR (14.8% versus 1.9% for EVAR, p<0.01). We found a trend towards higher reintervention rate in patients submitted to OSR (p=0.055), however it didn’t achieve statistical significance (Table 8).
Table 7 Comparison of demographic characteristics of patients undergoing urgent infrarenal aneurysm repair by EVAR or OSR, included in the AAA module of the Portuguese National Vascular Registry
| EVAR | OSR | p value | |
|---|---|---|---|
| n (%) | 110 (53.7) | 95 (46.3) | |
| Age - (mean years ± SD) | 77.38 ± 8.2 | 73.05 ± 10.5 | p = 0.001 |
| Male gender - n (%) | 97 (88.2) | 82 (89.1) | p = 0.833 |
| Aneurysm diameter - (mm ± SD) | 70.5 ± 21.5 | 80.4 ± 26.4 | p = 0.003 |
| Diabetes - n (%) | 21 (19.1) | 11 (11.6) | p = 0.139 |
| Ischemic cardiac disease - n (%) | 34 (31.2) | 23 (24.2) | p = 0.268 |
| Renal impairment - n (%) | 34 (32.1) | 31 (32.9) | p = 0.822 |
| Pulmonary disease - n (%) | 22 (20.2) | 11 (11.6) | p = 0.096 |
| Cerebrovascular disease - n (%) | 12 (10.9) | 9 (9.5) | p = 0.096 |
Table 8 Outcomes comparison of patients undergoing urgent infra-renal aneurysm repair by EVAR or OSR, included in the AAA module of the Portuguese National Vascular Registry
| URGENT SURGERY | EVAR | OSR | p value |
|---|---|---|---|
| Mortality | |||
| Intra-hospital mortality - n (%) | 29 (27.6%) | 38 (43.2%) | p = 0.024 |
| 30-day mortality - n (%) | 30 (28.8%) | 40 (44.4%) | p = 0.024 |
| Outcomes at 30 days | |||
| Myocardial infarction - n (%) | 10 (9.5) | 14 (15.9) | p = 0.181 |
| Cerebrovascular event - n (%) | 0 | 0 | |
| Pulmonary complication - n (%) | 24 (22.9) | 29 (32.9) | p = 0.117 |
| Renal impairment requiring hemodiafiltration - n (%) | 17 (16.2) | 16 (18.2) | p = 0.714 |
| Bowel ischemia - n (%) | 2 (1.9) | 13 (14.8) | p < 0.001 |
| Abdomen compartment syndrome - n (%) | 12 (11.4) | 9(10.2) | p = 0.790 |
| Abdomen compartment syndrome requiring laparostomy - n (%) | 9/12 (75) | 9/9 (100) | p = 0.105 |
| 30-day reintervention - n (%) | 12 (11.4) | 19 (21.6) | p = 0.055 |
| Hospital length - (mean days ± SD) | 14.62 ± 18.2 | 20.8 ± 3.4 | p = 0.133 |
| Blood products need | |||
| Red blood cell transfusion - (mean units ± SD) | 3.1 ± 3.4 | 5.3 ± 4.1 | p < 0.001 |
| Plasma transfusion - (mean units ± SD) | 1.1 ± 1.98 | 3.33 ± 3.4 | p < 0.001 |
| Outcome at 1 year | |||
| Survival status - n (%) | 23/41 (56.1) | 16/41 (39.0) | p = 0.122 |
In the urgent setting, the mean age of patients who survived at 30 days was 72.8 ± 9.0 years, compared with 79.6 ± 9.2 years for patients who died (p<0.01). Patient sex, history of cardiac, pulmonary, cerebrovascular disease, diabetes or previous aortic surgery were not found to significantly influence post-operative mortality. However, the presence of pre-operative renal impairment negatively influenced patient survival (p=0.001).
Concerning the anatomic features of the aneurysm, we identified a significant difference in the mean aneurysm diameter between survivors and non-survivors of 71.9 ± 24.1mm and 81.5 ± 24.3mm, respectively (p=0.009). Table 9 summarizes intra- and post-operative predictors of mortality.
Table 9 Predictors of mortality, in univariable analysis, of patients undergoing urgent infra-renal aneurysm repair by EVAR or OSR, included in the AAA module of the Portuguese National Vascular Registry
| Mortality rate (%) | p value | |
| Suprarenal clamp / aortic occlusion balloon | ||
| used vs. not used | 55,6 vs. 31,6 | p = 0.007 |
| 30-day myocardial infarction | ||
| present vs. absent | 58.3 vs. 32.1 | p = 0.012 |
| 30-day renal impairment requiring hemodiafiltration | ||
| present vs. absent | 66.7 vs. 28.9 | p < 0.001 |
| 30-day abdominal compartment syndrome | ||
| present vs. absent | 57.1 vs. 32.7 | p = 0.027 |
| 30-day pulmonary failure | ||
| present vs. absent | 71.7 vs. 21.6 | p < 0.01 |
vs.: versus
A multivariable analysis was subsequently performed, resorting to a logistic binary regression. All significant predictors of mortality in univariate analysis were included. Age (p<0.001) and 30-day pulmonary failure (p<0.001) were found to be independent risk factors for mortality.
Abdominal compartment syndrome (ACS)
Considering all the study population, ACS was identified in 2.5% (n=25) of patients, 88% of whom were submitted to urgent laparostomy. It was much more frequent in the urgent setting (10.8% versus 0.5%, p<0.001) and associated to a dismal prognosis. Cardiac, renal and pulmonary complications were significantly more frequent, culminating in an increase of mortality.
Impact of the caseload in the outcome
We divided the centers in four groups, according to the number of cases introduced per year in the AAA RNPV. In elective cases, intra-hospital mortality was inversely related with the caseload (p=0.032). OSR mortality was responsible for this reduction of mortality in centers with higher volume (p=0.04). The center caseload did not impact the intra-hospital mortality in elective standard EVAR procedures.
No significant relationships were observed in urgent cases (Table 10).
Table 10 Relationship between intra-hospital mortality and annual center volume of infra-renal aneurysm repair, in elective and urgent setting, included in the AAA module of the Portuguese National Vascular Registry
| Intra-Hospital Mortality | Total | OSR | EVAR |
|---|---|---|---|
| ELECTIVE SURGERY | |||
| <10 cases / year - n (%) | 7 (5.8) | 4 (18.2) | 3 (3.0) |
| 10-20 cases / year - n (%) | 0 (0) | 0 (0) | 0 (0) |
| 21-30 cases / year - n (%) | 3 (1.3) | 3 (4.4) | 0 (0) |
| >30 cases / year - n (%) | 7 (2.0) | 3 (3.2) | 4 (1.6) |
| p = 0.032 | p = 0.04 | p = 0.150 | |
| URGENT SURGERY | |||
| <5 cases / year - n (%) | 11 (34.4) | 10 (52.6) | 1 (7.7) |
| 5-10 cases / year - n (%) | 8 (25.0) | 3 (27.3) | 6 (28.6) |
| 11-15 cases / year - n (%) | 23 (50.0) | 10 (62.5) | 13 (43.3) |
| >15 cases / year - n (%) | 25 (30.1) | 15 (35.7) | 10 (24.4) |
| p = 0.077 | p = 0.157 | p = 0.084 |
Discussion
The present study reveals the results of the first 1000 infrarenal aneurysms included in the Portuguese AAA registry, giving novel clinical insights about the treatment of this pathology in our country.
A previous study evaluating data from hospital admissions within the Portuguese National Health Service in Continental Portugal, based on the hospital codification (Homogenous Disease Group - GDH), showed an increase in the number of hospitalizations due to AAA from 656 in 2009 to 896 in 2017.6) More than 600 registered cases per year were to be expected. However, our annual reporting was around 350 registries per year, raising the concern of substantial underreporting in the Portuguese RNPV. High compliance is of the utmost importance for reliable data, as patients not reported in the registries may present worse outcomes and create a selection bias.7
Additionally, regular audits of data abstraction are necessary to improve data quality, assure data validity and reliability and guarantee the integrity and credibility of registry outputs. Through these efforts, registries can improve quality of care by evaluating clinical practice, using reliable data. Cooperation in the Vascunet network can be an important evolution, given the possibility of direct comparisons with registries from other countries. Identification of differences can stimulate improvements in care and promote scientific projects.8)
In our study, AAA treatment was performed for diameter thresholds below the recommended by the ESVS guidelines in 16.3% of the patients, the majority of them in an elective setting (83.8%). Some cases might have been adequately performed due to saccular morphology or aneurism rapid growth. Although indication for treatment is a mandatory variable in the RNPV, a field for descriptional data is not available. Consequently, data was scarce and no rigorous conclusions can be made. In the Swedvasc, the number of procedures for AAA of less than 55mm was 20.5%12, in the UK registry it was 9.2%9 and in the DSAA it was 13.5% for EVAR and 12.1% for OSR.3 In a VQI evaluation, between 2013 and 2017, 22975 patients were included and 41% of the EVAR procedures were performed for small AAA (<5.5/5.0mm, according to diameter threshold of ESVS guidelines).1 From further analysis of this subgroup and comparing results with subgroups presenting with medium (5.5/5.0mm-6.5mm) and large AAA (>6.5mm) it was possible to conclude that type I endoleak at completion angiography, reintervention and the mortality rate at 1 and 5 years enhanced with increasing diameter (p<0.001).10)
We should analyze these results with caution, avoiding overtreatment of AAA that may remain stable for a long time. Furthermore, one might consider that patients with large AAA have less prior exposure to vascular care and poor risk factor management, that can negatively influence early and late results.10 National AAA screening in at-risk populations for AAA development may be a useful tool. Also, adherence to a strict imaging follow-up regimen in identified AAA is of paramount importance and not infrequently requires special emphasis by the attending surgeon.
According to our data, the 30-day mortality for elective repair is 1.8% in EVAR group and 5.9% in OSR group. A recent meta-analysis, integrating data from EVAR-1, DREAM, OVER and ACE trials, comparing outcomes of elective EVAR or OSR for AAA, identified a lower total mortality in the EVAR group only in the first 6 months of follow-up (3.3% versus 5.3% deaths).11) However, owing to the rapid technological and medical developments, RCTs are partly outdated and thereby not entirely valid for our current reality.
The contemporary 30-day mortality with elective EVAR is around 1%, compared with a three to four times higher mortality after OSR.1) In Swedvasc, 30-day mortality was 1.1% for EVAR and 2.5% for OSR.12 In DSAA, peri-operative mortality was 1.3% and 4.6% for elective EVAR and OSR, respectively.3)
The DSAA, evaluating treatment outcome trends from 2014 to 2019, found that peri-operative mortality and major complications have improved over time, except for the peri-operative mortality following EVAR which remained unchanged.3)
The most recent paper published by the ICVR, including over 100 000 intact AAA treated throughout 11 countries, from 2010 to 2016, identified a reduction in peri-operative mortality for both elective EVAR and OSR (0.7% and 3,6%, respectively).13) Poorer outcomes were identified in women and octogenarians.
Our data are unfortunately too recent to verify temporal trends and we only identified the baseline AAA diameter as a predictor of 30-day mortality.
These data confirm that elective EVAR, in Portugal, compares with the results in the rest of the western world. The higher mortality with OSR can be partially explained by the changing in AAA treatment over time, favoring EVAR. In centers with lower caseload of elective AAA treatment, we identified a risk of intra-hospital mortality with OSR significantly higher. A strong volume-outcome relationship for OSR is published in the literature, with high-volume hospitals associated with lower perioperative mortality.14) Exposure to OSR among vascular surgery training programs has dramatically decreased over time in the portuguese and world reality, which raises a growing concern surrounding competence of younger surgeons with this technique.14,15
This should be a subject of national reflection and should serve as a driver for adjustments in training programs. Clear technical standards, redefined volume and case-mix thresholds, systematic performance measurement and leverage quality collaboratives, such as the VQI, may be necessary to ensure that trainees are ready for independent aortic practice when entering the workforce.16-18
To date, in the Portuguese RNPV, there is a lack of information regarding preoperative medication and prior management of cardiovascular risk factors. Our post-operative complications seem to mirror the results of other registries.2,3
Several studies suggest that the 30-day mortality is not the best quality indicator and advise to use long-term survival (minimum, one year) and reintervention rate.12) Results at one-year are only available for 30% of our sample, substantially due to underreporting. Once more, we underline that more data of the Portuguese RNPV are needed to identify opportunities for improvement in elective AAA treatment in our country.
Roughly one fifth (n=205, 20.5%) of the infrarenal aneurysms in the Portuguese RNPV were treated urgently, of which 137 due to AAA rupture. The significantly larger AAA-diameter in the urgent cases (70.5 ± 21.5 mm versus 57.5 ± 14.3 mm, p < 0.001) call for action regarding the establishment of an AAA-screening program in Portugal.
We identified a small preponderance of EVAR comparing to OSR in the urgent setting (53.7% versus 46.3%), which is not in line with the literature.19-21) The VQI reported an increase use of EVAR in ruptured AAA, from 7.8% in 2004 to 67.2% in 2018, with favourable short- and long-term morbidity and mortality, as compared with OSR.19) This EVAR-first approach, supported by ESVS guidelines1, is being increasingly used. Current literature, based on real-world experience, supports a significant lower mortality with EVAR in the urgent setting, with some centers adopting an EVAR-only approach.20,22
In our study, the small preponderance of EVAR in the urgent setting might be a consequence of logistical limitations or team training, but it is difficult to isolate the type of patients treated at each center.
The 30-day mortality, in urgent procedures was 28.8% and 44.4% in EVAR and OSR groups, respectively. Mortality rates after EVAR for ruptured AAA vary in the literature and range from 13% to 53%.1) In Swedvasc, mortality after ruptured AAA repair was 21.2% and 28.1%, for EVAR and OSR, respectively.12 We identified several factors that may compromise the outcome of urgent AAA treatment, which are in line with the literature: age, pre-operative renal impairment, aneurysm diameter and anatomy. Post-operative myocardial infarction, hemodiafiltration dependence, pulmonary failure and ACS also had a negative impact on the outcome. Resorting to multivariable statistics, it was possible to identify age and 30-day pulmonary failure as independent risk factors for mortality.
In our study, no significant relationships were observed between intra-hospital mortality and center caseload, in the urgent setting. These results may be biased and reflect institutional and referral health policies. Some of the included centers do not have a 24/7 urgent care. Consequently, hospitals with a 24/7 vascular surgery care available receive patients from a larger referral area, with some patients being transferred with longer rupture times and treated in a more unstable condition.
ACS is an early, devastating complication. In our study, in the urgent setting, it was diagnosed in 11.4% and 10.2% of the patients in the EVAR and OSR groups, respectively. In Swedvasc, it was identified in 6.9% and 6.6% after urgent EVAR and OSR, respectively.12) Similar to our findings, decompression laparotomy was frequently performed (Portugal: 75 - 100%; Sweden: 77.3 - 84.6%).12 Even so, prognosis was very reserved, which highlights the importance of a protocol algorithm, ideally based on the ESVS guidelines, for early screening and diagnosis and expeditious management.1
Our study has some limitations, the main being the low reporting of mid to long-term follow-up data. Furthermore, there is a potential case for selection bias, since the database is of voluntary registration. Additionally, centers volume was assessed based only on registry data. We highlight that there is a possibility of information bias conditioning this assessment, given the voluntary participation in the registry. So far, no internal validation audit has been conducted.
Conclusion
Vascular registries reflect real-world practice and offer the advantage of rapid feedback, which is of paramount importance when new technical developments are frequently introduced, as in the case of the treatment of AAA. In this report of 1000 patients treated for infra-renal aneurysms, results are generally favourable and comparable to existing literature from other countries in Europe and North America.
Acknowledgements
This report would not have been possible without the continuous registration efforts over the years of the past and present Principal Investigators and Sub-investigators of each center.
Conflicts of interest
Clara Nogueira has received speaker fees from W. L. Gore.
Frederico Bastos Gonçalves has received speaker and proctoring fees from Medtronic AVE, Cook Medical and W. L. Gore.
Luís Mendes Pedro has received speaker and proctoring fees from Cook Medical, W. L. Gore and Terumo Aortic.















