Introduction
Customized endografts, namely f/bEVAR, present lower perioperative complications compared to open repair, in complex aortic pathologies. Open repair, particularly in thoracoabdominal aortic diseases, remains one of the major challenges in vascular surgery and is associated with high rates of major complications and in-hospital mortality rate of up to 32%.1) Comparing to open repair, f/bEVAR presents a lower mortality rate (as low as 5.8%).2) Despite early mortality and major complications benefit, f/bEVAR requires a long waiting time for customized graft production. Despite availability of off-the-shelf branched solutions, they are also limited by specific anatomic features. Alternatively, adapting off-the-shelf commercially available devices, which are readily available and can surpass these anatomic restraints, has been used with variable success. The Octopus technique is one of these strategies, where a standard bifurcated EVAR is deployed in the thoracic aorta and up to four parallel bridging stent-grafts are used to perfuse visceral branches.3,4) Despite an off-label combination of commercial devices, it can play a role when f/bEVAR is unavailable or unsuitable. This study aims to evaluate short and midterm outcomes of the Octopus endograft technique in the treatment of complex aortic pathologies.
Methods
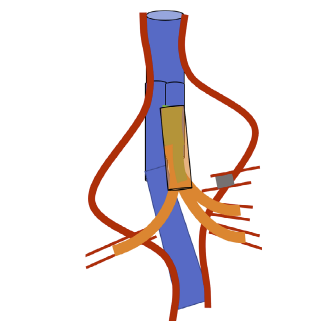
This study is in accordance with institutional policy for observational clinical investigation and the Declaration of Helsinki. It is a single center retrospective study, conducted in a Portuguese tertiary care university center. All consecutive patients treated with the Octopus Endograft Technique since 2015 until February 2022 were included. Figure 1 depicts a schematic example of the technique.
Representation of a three-branch Octopus. The main body of the stent graft is represented in blue. The bridging stent grafts are represented in orange. In this example, the coeliac trunk was chronically occluded, so only three branches were required.
Both primary and secondary endovascular aortic procedures were allowed. Urgent and elective cases were included. Patients were identified through institutional files. Baseline characteristics (age, gender, comorbidities, medications, ASA score), clinical presentation (asymptomatic, symptomatic, ruptured), aortic pathology (aortic diameter, aneurysm anatomy, previous aortic intervention), procedural data (anesthesia, surgical access, procedure duration, blood loss, contrast use, intraoperative complications and adjunctive procedures), early and mid-term post-operative outcomes (complications, reinterventions, death), concerning procedure-related complications and death were obtained. Primary endpoint is clinical and technical 30-day success. Secondary endpoints are complications and re-interventions in all follow-up. Descriptive statistics were used to report data. Normally distributed data is presented as mean (standard deviation) and non-normally distributed data as median (interquartile range). Kolgomorov-Smirnov and Shapiro-Wilk tests were used to assess distribution normality of data using SPSS version 26.0 software.
Results
Between May 2015 and February 2022, six patients (50% male; 50% female) with mean age of 74 (±9) years treated with this technique were identified. Baseline characteristics are depicted in Table 1. Most common associated comorbidities were hypertension (n=5), hyperlipidemia (n=5) and cardiac disease (n=4). Four patients were under beta-blockers and all patients presented with an American Society of Anesthesiology score (ASA) ≥3. Three patients (50%) had a previous thoracic (TEVAR) (n=1) or abdominal (EVAR ± proximal extension cuff) endovascular aortic intervention. Four procedures were elective and the remaining emergent (n=2). Indications for treatment (Table 2) included three type 1 endoleaks (EL), of which one was ruptured, and three thoracoabdominal (TA) aortic aneurysms without prior intervention, one of which was a ruptured mycotic aneurysm (Figure 1).
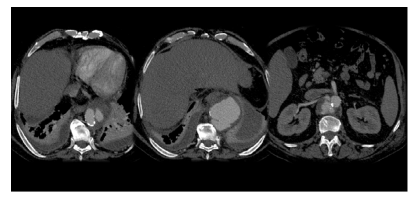
Figure 2 Pre-operative computed tomography angiography of a mycotic thoracoabdominal aneurysm, treated with the Octopus technique
Table 1 Baseline characteristics of patients treated with the Octopus technique
| Baseline Characteristics | Total |
| Age in years, mean (SD) | 74.3 (±9) |
| Male, n(%) | 3 (50%) |
| Smoking History, n(%) | 3 (50%) |
| Hypertension, n(%) | 5 (83%) |
| Hyperlipidemia, n(%) | 5 (83%) |
| Heart Disease, n(%) | 4 (67%) |
| Cerebrovascular disease, n(%) | 1 (17%) |
| Pulmonary disease, n(%) | 2 (33%) |
| PAD, n(%) | 3 (50%) |
| CKD without dialysis, n(%) | 2 (33%) |
| CKD on dialysis n(%) | 2 (33%) |
| Beta-blocker, n(%) | 4 (67%) |
| Statin, n(%) | 3 (50%) |
| Antiplatelet, n (%) | 3 (50%) |
| Anticoagulant, n (%) | 2 (33%) |
| ASA ≥3, n (%) | 6 (100%) |
| Aneurysm diameter in mm, mean (SD) | 62 (6.7) |
SD: standard deviation; PAD: peripheral artery disease; CKD: chronic kidney disease; ASA: American Society of Anesthesiology
Table 2 Aortic Pathology of patients treated with the Octopus technique
| Total | |
| Urgent, n(%) | 2 (33%) |
| Elective, n(%) | 4 (67%) |
| Previous aortic intervention, n(%) | 3 (50%) |
| -TEVAR + coil embolization celiac artery | 1(16%) |
| -AUI EVAR + femoral crossover + proximal aortic extension cuff | 1(16%) |
| -EVAR + IBD + limb extension + proximal Palmaz stent | 1(16%) |
| Pathology n(%) | |
| - Ruptured mycotic aneurysm | 1 (16%) |
| - rEVAR due to type 1a endoleak | 1 (16%) |
| -Type 1a EL post-EVAR | 1 (16%) |
| -Type 1b EL post-TEVAR | 1 (16%) |
| -Type 4 TAAA | 1 (16%) |
| -Type 5 TAAA | 1 (16%) |
AUI: aorto-uni-iliac; IBD: iliac branch device; EL: endoleak
Mean pre-operative maximum aortic diameter was 62 (±6.7) mm. In the elective setting, this technique was chosen in 3 patients due to anatomical constraints and in the other one due to limited life expectancy. In all cases, the rupture risk was considered very high, or signs of impending rupture were noted. This technique was used in emergent procedures due to unavailability of b/fEVAR. All procedures were performed under general anesthesia. Prophylactic cerebrospinal fluid (CSF) drain was used in one emergent procedure. Bilateral femoral and left axillary access was obtained in all patients, (Table 3). GORE® Excluder™ and CORDIS® Incraft™ bifurcated aortic endografts were used in five and one cases, respectively. Thirteen visceral branches were revascularized with GORE® Viabahn™ stent grafts (6 superior mesenteric artery (SMA), 4 renal and 3 celiac), with no failed branch graft implantation attempts (Table 4). Branches not revascularized were due to known chronic occlusion, previous embolization or coverage or because distal sealing was proximal to their origin. Final angiography showed gutter EL in 2 patients, which were not further addressed intraoperatively (Figure 3). One resolved spontaneously, the other required reintervention.
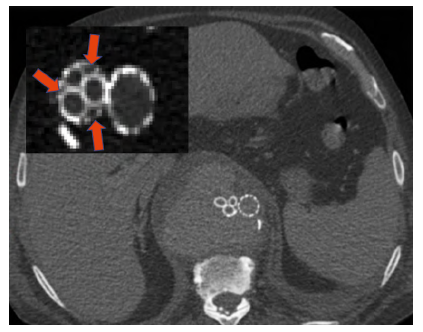
Figure 3 Example of gutter endoleaks, due to insufficient oversizing. Red arrows showing the gutters without adequate stent apposition
Table 3 Procedure related characteristics of patients treated with the Octopus technique
| Procedure related characteristics | Total |
| General Anesthesia, n(%) | 6 (100%) |
| CSF drain, n(%) | 1 (17%) |
| Blood Loss, Mean(SD) (mL) | 483 (300) |
| Surgery time, Mean(SD) (min.) | 288 (73) |
| Contrast, Mean(SD) (mL) | 120 (57) |
| Fluoroscopy, Mean(SD) (min) | 51 (40) |
| Percutaneous, n(%) | 2 (33%) |
| Conversion, n(%) | 0 (0%) |
| Adjunctive procedures, n(%) | 2 (33%) |
| Introperative complication, n(%) | 2 (33%) |
CSF: cerebrospinal fluid. Percutaneous means that all accesses were percutaneous.
Table 4 Individual procedure data of patients treated with the Octopus technique
| Patient | Endograft | Branches Revascularized | Other Branches | Intraoperative Endoleak |
| 1 | Gore Excluder | SMA + RRA + LRA | CA occluded | Gutter |
| 2 | Cordis Incraft | SMA | CA + RRA + LRA occluded | No |
| 3 | Gore Excluder | SMA + RRA + LRA | CA previously embolized | No |
| 4 | Gore Excluder | CA + SMA | RRA + LRA occluded | Gutter |
| 5 | Gore Excluder | CA + SMA | Distal sealing above renals | No |
| 6 | Gore Excluder | CA + SMA | RRA + LRA covered | No |
SMA: superior mesenteric artery; RRA: right renal artery; LRA: left renal artery; CA: celiac axis.
Table 5 Individual procedure data of patients treated with the Octopus technique
| 30-day outcomes | |
| ICU stay, mean (SD), in days | 6 (2.7) |
| Hospitalization, mean (SD), in days | 26(19.5) |
| Acute renal injury, n(%) | 2 (34%) |
| Infection, n(%) | 2 (34%) |
| Pulmonary complications, n(%) | 1 (16%) |
| Cardiac complications, n(%) | - |
| Paraplegia, n(%) | 2 (34%) |
| Other neurologic complications, n(%) | 1 (16%) |
| Postimplantation Syndrome, n(%) | 1 (16%) |
| Branch compression, n(%) | 1 (16%) |
| Branch thrombosis, n(%) | - |
| Reintervention, n(%) | 2 (34%) |
| Mortality, n(%) | 1 (16%) |
| Antiplatelet, n (%) | 3 (50%) |
| Anticoagulant, n (%) | 2 (33%) |
| ASA ≥3, n (%) | 6 (100%) |
| Aneurysm diameter in mm, mean (SD) | 62 (6.7) |
SD: standard deviation
Two axillary hemorrhages after percutaneous access were noted and treated with stent-grafts. Mean (SD) blood loss, surgery time and contrast use were 483(±300) mL, 288(±73) min and 120(±57) mL, respectively. Mean (SD) hospitalization time was 26(±19.5) days (Table 3). Most frequent postoperative complications were acute renal failure (n=2), paraplegia (n=2) and infection (n=2). Paraplegia occurred in one patient with prophylactic CSF drain and in one patient without. In the last, rescue CSF drainage resolved motor and sensitive deficits. Infection of axillary surgical site and bacteremia occurred in 1 patient each, successfully treated with antibiotics. Two patients had reinterventions in the first 30 days: one relining with bare-stent due to renal branch compression and gutter coil embolization; and one axillary hematoma drainage. One perioperative death (16%) occurred, in a patient emergently treated for a secondary rupture (type 1A EL), due to hemorrhagic shock. (Table 5). On follow-up, with a median (IQR) time of 13 (2-18) months, there were no new EL nor reinterventions, and all branches remained patent. During follow-up, four patients died one with an infection-related complication (spondylodiscitis in a patient with a thoraco-abdominal mycotic aneurysm (Table 6). The remaining deaths were not aneurysm related.
Discussion
In thoraco-abdominal (TA) aortic pathologies, decision-making process is complex with respect to operative strategies and perioperative management. Nowadays, we observe increasing rates of late EVAR complications, particularly EL. In these patients, due to their multiple comorbidities, the risks of treating these complications with open surgical techniques may be prohibitive.5,6
Endovascular therapy with branched or fenestrated grafts is now being increasingly used to address com (plex TA pathologies. These solutions, however, are not universal, being limited by certain anatomic features and require time for customized graft production. There are also off-the-shelf branched solutions for TAAA, such as Zenith® t-Branch® TAAA stent-graft (Cook Medical, Bloomington, IN, USA), GORE EXCLUDER thoracoabdominal branch endoprosthesis (TAMBE, W.L. Gore & Associates Inc, Flagstaff, AZ) and E-nside® TAAA Multibranch Stent Graft System (Jotec GmbH, Hechingen, Germany).7) Only the Zenith t-Branch® was available in Portugal during the study period, while the TAMBE® is still under investigation and the E-nside® only recently has become available.
A systematic review and meta-analysis of branched endografts (t-Branch) for the treatment of TAAA, including 187 patients, found a pooled technical success of 92.75% (95% CI, 83.9%-98.7%) and early mortality of 5.8% (95% CI, 2.5%-10%). The rate of spinal cord ischemia was 12.2% (95% CI, 4.1%-23.2%). Acute renal failure was 18.7% (95% CI, 9.1%-30.4%), whereas primary branch patency was calculated at 98.2% (95% CI, 96.7%-99.2%). Midterm mortality was 6.9% (95% CI, 2.44%-12.8%) and reintervention rate was 5.7% (95% CI, 1.70%-11.4%).2) Despite good results and being readily available, a narrow aortic lumen (below 24mm), narrow access vessels (below 8.5mm), distance from branch to vessel origin (greater than 50mm) or neck angulation greater than 90 degrees limit its use.
In such instances, adaptation of commercially available off-the-shelf devices is increasingly described in techniques such as chimneys, snorkels, periscopes, sandwich or the Octopus technique. First described by Kasijaran and later modified by Silveira et al, the Octopus technique uses a standard bifurcated main body in the descending thoracic aorta.3,4) Following this, three Viabahn® stent-grafts (W.L. Gore & Associates Inc, Flagstaff, AZ) are deployed inside the short leg (13-mm diameter) through an axillary conduit. These Viabahn® stent-grafts are subsequently extended to the celiac trunk, superior mesenteric artery, and right renal artery. In the remaining long limb of the Gore Excluder® bifurcated endograft (W.L. Gore & Associates Inc, Flagstaff, AZ), a Viabahn® and a parallel bell-bottom extension are deployed. The bell-bottom is connected to a new bifurcated device, and the Viabahn® is extended to the left renal artery. The long limb was placed 2-3 cm above the highest visceral branch intended to be preserved.
In our series, there was 100% visceral branch cannulation with 13 visceral GORE® Viabahn™ stentgraft implants (6 SMA, 4 renal and 3 celiac). The remaining visceral vessels where intentionally not revascularized due to chronic occlusion, previous embolization or because distal sealing was proximal to their origin. In one case (emergent secondary rupture), there was previous renal arteries coverage with an uncovered stent, which made them impossible to catheterize, and the patient was on pre-dialysis status. In our series, there were no exact reproductions of the original modified technique due to concomitant visceral vessel disease. Also, we always used an axillary sheath instead of a conduit. In 3 patients, there was 2 vessel and in 1 there was single vessel revascularization. In 3 vessel revascularization procedures, the short limb was used as a gate for all visceral branches and a combination of 7mm (2 stents) and 8mm stents was used; in the other patient, a combination of two 6 and a 9mm stent was used. According to Silveira et al, a combination of 8mm and 7mm or three 7-mm stentgrafts presented the best conformability and juxtaposition minimizing gutter endoleaks. In the case of 6mm grafts, there was an unfavorable juxtaposition and higher gutter space, independently of post-dillation.12)
Concerning patient selection, our patients were elder, with a mean age of 74.3(±9) years, with some degree of dependency on daily activities and with high rates of cardiovascular comorbidities. Their extensive comorbidities and impaired ability to sustain a high surgical stress precluded consideration for open repair. Concerning procedure choice, in the two urgent cases (mycotic TAAA and rEVAR due to type 1a EL after EVAR and proximal Palmaz stent) the Octopus technique was chosen based on f/bEVAR unavailability and narrow aortic lumen. On the remaining patients, key factors justifying the procedure were a combination of anatomic constraints and limited life expectancy, while there were signs of impending rupture, or the rupture risk was considered to be very high. Another important motive justifying the use of this technique was the ability to limit aortic coverage and the risk of spinal cord ischemia, particularly in one case, where distal sealing was obtained above the renal arteries. Concerning graft selection, as the original modified technique, bifurcated Gore® Excluder® endografts (limb diameter 13mm) were chosen in 5 patients. (Table 4) In one patient a Cordis® Incraft® bifurcated endograft was chosen. This was related with its narrower limb diameter (11mm) since this patient had associated extensive occlusive visceral disease, with only a patent SMA, as this was easier to accommodate one visceral branch only. Other than Gore® Excluder® grafts, there are also reports of successful use of Medtronic® Endurant® graft. Its larger limb diameter (14mm) presents a theorical advantage of providing a larger conduit for visceral blood flow, but with a concern of an increased risk of type 3 gutter endoleak.8,9
In other groups, as in our series, 4 branch revascularization was not frequent, with the majority of patients having 3 branches revascularized, being the most common sacrificed vessel the celiac axis.9,10,11) This was due a combination of chronic occlusion, severe stenosis with good collateral pathway or inability to cannulate. Concerning 2 branch revascularization, in our series just 2 stent-grafts were deployed via the short-limb. In this scenario, Hsu et al proceeded to implant three branches in the short-limb of the bifurcated graft and selectively embolized one with a plug or coils, in order to minimize the risk of a gutter EL.9) Other groups do not report technical considerations regarding 2 vessel revascularization. Overall technical success was above 90% in all series.
Regarding vascular access, besides femoral access, axillary/brachial access was used in all patients. In contrast to the original technique, we did not use an axillary conduit, and direct arterial puncture was used in all patients. In two cases, percutaneous axillary access was obtained, both with access site hemorrhage, treated successfully with stent-grafts. In these cases, we routinely had a distal ipsilateral brachial artery access that permitted rapid control of the hemorrhage.
In our series, paraplegia ensued in two patients (33%). A prophylactic CSF drain was used in one emergent patient, and, despite that, he developed paraplegia. In this patient, refractory hypotension was the main reason for this complication. In the other patient, rescue CSF drain was able to reverse the neurologic deficits. In all series, prophylactic CSF drain was routinely used, although in two groups just after an initial experience without this adjunctive technique. Despite this protective procedure, paraplegia was frequent, with reported rates of 28.5% (Dua et al); 16.6% (Wooster et al) and 18.8% (Hsu et al), which is comparable to our 34%.9,10,11) These results present, however, worse than those reported with the use of the t-Branch device (12.2%).2
There are few cases reported of this technique, considered by many as a bailout strategy in no-option patients. Given its complexity in planning and execution, there is an expected learning curve, which can impact procedural details, such as time of surgery, blood loss, fluoroscopy and contrast use. Mean blood loss was 483mL in our series vs 807mL of Dua et al.11 Contrast use was also elevated with 120 mL (our series) vs 182 (Dua et al) vs 90mL (Wooster et al).10,11) Operative time for this procedure were long, with a reported 8h (Dua et al) vs 9h (Hsu et al) vs 5h (our series).9,11) Schanzer et al, analysing 893 patients from 6 prospective, nonrandomized studies evaluating f/bEVAR between 2012 and 2018 in the US, found a median of 4.6 to 5.2 hours of surgery in f/bEVAR with a mean of 3.5 fenestrations/directional branches and 97 to 111 mL of iodinated contrast use, depending of previous infrarrenal EVAR. These results show that Octopus technique is probably more time consuming and contrast demanding procedure.13
In the first 30 postprocedural days, complications were common. However, most were not endoprosthesis related (pulmonary, renal, bowel, neurologic). In our series, one patient presented a major gutter EL and renal branch compression, requiring gutter coil embolization and branch relining with self-expandable stent. Other groups report variable frequencies of endoprosthesis complications, namely EL and branch-related complications. Hsu et al report intraoperative endoleaks in 5 patients, however without further procedures.9 We note that in their series 3 patients died early and further follow-up showed EL resolution on the remaining. Wooster et al noted EL in 4 patients, 3 gutter EL and one type 2 EL.10) One SMA branch thrombosis occurred and required thrombectomy, stent relining and bowel resection. Dua et al had 5 endoprothesis-related early reinterventions due to EL or branch stent complication.11
Mortality is reportedly elevated after this procedure, with 1 (16%) case in our series, comparing to 27.7%; 25%; 14.3% in others, which present a higher mortality than after a t-Branch device.9,10,11 Our patient expired due to multiorgan failure after emergent surgery due to secondary rupture. Dua et al and Wooster et al reported both 1 death related to SMA stent thrombosis and acute mesenteric ischemia and the remaining from miscellaneous causes.10,11) These results present unfavorable when comparing to an off-the-shelf branched solution (pooled reintervention rate of 5.7%; 95% CI, 1.70%-11.4% and midterm mortality of 6.9%; 95% CI, 2.44%-12.8%).2
Hospitalization time also seem higher in our group of patients, comparing to b/fEVAR devices: 26 days vs 6.8 days. 13
On mid-term follow-up, endograft related complications were less frequent and no patients required reintervention after 30 days. One gutter EL was proposed to imaging follow-up and disappeared. We also report 1 case of persisting infection resulting in spondylodiscitis in a patient previously treated for a mycotic aneurysm and he eventually expired due to this motive. All branches were patent up to last follow-up. Other groups reported long-term branch patency of 89-100%. Hsu et al reported 2 cases of late renal branch thrombosis and no type 1 or 3 endoleaks in mid-term.9Wooster reported 1 late limb disconnection that underwent successful relining.10) Late mortality rates, conversely, appeared high. At two years we obtained a mortality rate of 86%, comparing unfavorably to 48% in Dua et al.’s report.11
In summary, one of the major advantages of this procedure is its availability in urgent scenarios and in narrow, severely angulated or tortuous aortas, as in those with extensive occlusive disease or infective aortic diseases. The possibility of being able to revascularize every combination of visceral branches also seems appealing. The main drawbacks are the longer operative time, higher use of radiation and contrast, and the described high need for early reinterventions, suggesting that thorough planning and follow-up are required. The complications and mortality associated are also substantial. On contrary, f/bEVAR can shorten catheterization distance, provide strong support, potentially reduce operation time and radiation exposure, at the same time allowing for staged procedures. It also allows an early closure of the femoral access´, thereby reducing the risks of lower extremity ischemia. The technical challenges and applications of the Octopus technique demand proficiency in endovascular planning and execution, which takes a considerable learning curve. The high mid-term mortality rate suggests that we are treating a very ill group with limited life-expectancy, suggesting that possibly there may be a group of patients who benefit of a “simplified” treatment strategy that can safely avoid aneurysm-related mortality without limiting quality of life. Also, the potential benefit of a non-interventional approach in these very diseased patients pose a significant decision challenge.
Conclusion
The octopus technique offers a valuable off-the-shelf solution for patients with complex aortic pathologies, particularly due to anatomical constraints or emergent setting, limiting the use of customized grafts. Despite a high technical success rate, there is a learning curve to consider and significant early morbidity and high mid-term mortality in a frail group of patients. In our small series, durability was reasonable with no need for secondary interventions. Our outcomes are in accordance with other reported outcomes.
Acknowledgements: None
Conflicts of interest: None
Funding: None