Introduction
Owing to advancements in stent graft design and involved technology along with improvement in experience of surgeons, endovascular repair of aortic aneurysms (EVAR) has become the most commonly used technique for treatment of abdominal aortic aneurysms (AAA) in most vascular surgery centres worlwide1 and including in Portugal.2 Despite this, secondary aortoenteric fistula (AEF) after EVAR is still a poorly studied complication, probably due to its low incidence.3 As there is still a lack of guidelines and quality evidence on this topic, our aim was to review available information regarding key aspects on post EVAR AEF, such as aetiology, clinical presentation, diagnosis, management strategies and prognosis.
Methods
We conducted a non-systematic MEDLINE literature search using the following search terms: “secondary aortoenteric fistula”, “endovascular aneurysm repair” and “abdominal aorta aneurysm”. Only English literature was considered. No specific time period was predefined. Additional articles found to be of interest for the purpose of this narrative review were also included by cross-referencing. Information was gathered and summarized by topic: aetiology, clinical presentation, diagnosis, treatment and prognosis.
Results
Secondary AEF is a well-known complication after aortic interventions, particularly open aortic repair. However, AEF after endovascular repair is less well studied. It was first reported in literature by Norgren et al. in 1998.4 Since then, its incidence has been hard to calculate due to lack of literature, underdiagnosis or loss of follow-up. The largest study on this topic is an Italian multicentric study by Kahlberg et al., which reported a lower rate of AEF after EVAR (<0.5%) when compared to open aortic repairs (up to 1.6%).3
Aetiology
Causes for AEF after EVAR can be classified according to three main categories, which should be taken in consideration, either isolated or in combination. The first factor is local infection, which may be pre-existing, as in the case of mycotic aneurysms, or secondary to the EVAR procedure itself. AEF is more likely to occur after EVAR for inflammatory aneurysms.3,5-8 Additionally, in the case of non-inflammatory aneurysms, evidence also shows that the EVAR procedure itself may lead to inflammation of the aorta and its surrounding structures, which in consequence could lead to fistula formation.5-7
The second potential cause is mechanical factors associated with the aneurysm. The anatomical shape and size of the aneurysm, particularly in the case of significant tortuosity, may increase pressure and cause stress to the bowel wall due to friction that may result wall necrosis with consequent ulceration or perforation.3,5-9
Lastly, the third possible cause is mechanical factors associated with the stent graft, which may result in aortic wall erosion and erosion into adjacent viscera. Examples of such factors include stent graft kinking or migration, suture disruption, endoleak, endotension provoked by the stent graft or coil embolization.3,5-10
Clinical presentation
Timing for the occurrence of AEF after EVAR is highly variable. A review by Koda et al. refers an interval from EVAR to AEF diagnosis of 20.4 ± 17.5 months.7 Patients frequently present with non-specific symptoms, thus requiring a high level of suspicion. The most common symptoms include abdominal or back pain, nausea, fever and gastrointestinal bleeding.3,5,9 The initial bleeding episode is often self-limited due to the formation of thrombus, with more episodes occurring over a variable period of hours, days or weeks.6 Therefore, haemodynamic instability and haemorrhagic shock is only present at admission in less than 20% of patients. This low rate of severe presentations may also be due to less severe aortic bleeding in the setting of an adequately excluded AAA by EVAR without endoleaks, when compared to AEF after open repair.3
Commonly the clinical presentation may be more suggestive of chronic infection, with symptoms of prolonged fever and weight loss with no noticeable gastrointestinal haemorrhage, further hampering a prompt diagnosis.5
Diagnosis
Patients with a suspected AEF should be submitted to a laboratory work-up with full blood counts including acute inflammation markers, which are usually elevated. Additionally, blood and urine microbial cultures should also be collected to identify any other possible infection sources and to isolate any pathogen for direct antibiotic therapy before surgical samples can be obtained for culture.11
Computed Tomography (CT) angiography scan is the first-line imaging study performed in most patients in which an AEF is suspected, despite reports describing a highly variable sensitivity ranging from 33-100%.3,5 Characteristic findings include periprosthetic gas, periaortic fluid collections, thickening of the intestinal wall or direct visualization of the fistula itself (Figure 1).3,5,9,12 Contrast extravasation into the bowel and leakage of enteric contrast directly into the periaortic space, albeit highly specific signs, are only rarely found.12
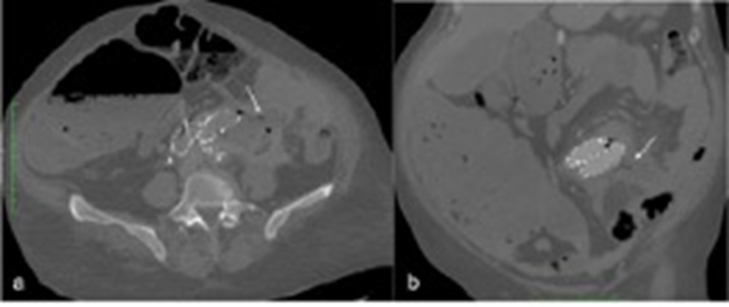
Figure 1 CT angiography showing a secondary AEF after EVAR (white arrow); gas around the prosthesis and periaortic fluid collection can also be seen - axial and coronal views (a, b).
Upper endoscopy may also be performed in the setting of gastrointestinal bleeding.3 Highly suggestive findings of AEF are bleeding arising from a point distal to the second part of the duodenum with no identifiable proximal lesions and visualization of an extrinsic, pulsating bleeding mass in the third portion of the duodenum.6 However, upper endoscopy failed to diagnose AEF in a quarter of patients in the previously mentioned study by Kahlberg et al.3 Moreover, it does not exclude the possibility of AEF if no fistula is seen.5
When CT scan is deemed inconclusive, a FDG-PET scan may be considered, as it is helpful in the diagnosis of vascular graft infections even if associated with a risk for false positives.3,13 Other lesser used imaging methods described in literature include angiography and capsule video endoscopy.5
Treatment
Treatment for post-EVAR AEF is challenging and involves total graft excision along with arterial reconstruction, bowel repair and antibiotic therapy.3,5,7 There are two main arterial reconstruction options: extra-anatomical reconstruction and in-situ reconstruction.
The first strategy involves infrarenal aortic ligation, total graft excision along with extensive infected tissue debridement and extra-anatomical revascularization usually through an axillo-bi-femoral bypass, either in a one or a two-staged procedure.3 A two-staged procedure should be considered in the case of stable patients since it is associated with a reduction of operative metabolic and haemodynamic stress as well as reduction in mortality and amputation rates.13
Complete endograft explantation is recommended, however, it may be difficult to perform, particularly in cases of endografts with suprarenal fixation, as its removal may lead to complications such as a tear in the aorta or injury to visceral branches and prolong suprarenal cross-clamp time. Several techniques have been described in this situation. First, one of the techniques involves the use of a 20mL syringe as a sheath to encircle the endograft and collapse its suprarenal component, leading to its removal without the above-mentioned risks. Secondly, the use of a wire cutter to release the metallic barbs from the main body of the endograft as also been described. Lastly, cutting the endoprosthesis with removal of all covered stents but leaving in place the suprarenal bare metal stent when it is strongly attached with the bards and hooks incorporated to the aortic wall. This last strategy is considered acceptable as bare metal stents are generally not an infection site.14
Extra-anatomical bypass is the preferable choice when there is a high level of contamination due to purulent fluid collection or gross retroperitonitis.3,7,10 Associated risks include aortic stump disruption with life-threatening haemorrhage (up to 27%), low patency rates of axillo-bi-femoral bypass with graft occlusion and high re-intervention rate, with consequent risk for amputation (as high as 75% at 5 years) and reinfection (up to 27%).13 To prevent aortic stump blow-out some techniques have proven to be helpful and can include double suture layers, reinforcement with pledgets, prevertebral fascia or a layer of peritoneum, reinforcement with bovine pericardium patch and omentoplasty.15
In-situ reconstruction is the other option for arterial revascularization.3 To reduce reinfection risk, antimicrobial impregnated grafts (rifampicin, silver acetate, triclosan), cryopreserved allografts, xenogenous grafts (bovine pericardium) or autogenous grafts (femoral vein graft created neoaorta - NAIS procedure) may be used.3,9 A meta-analysis by Batt et al. compared 5 different types of conduits used in in-situ reconstructions (PTFE grafts, rifampicin or silver coated polyesters, allografts or autogenous veins) and found that reinfection rates were similar between the different conduit options and no conduit showed any particular advantage in the presence of a prosthesis-duodenal fistula15 Silver/rifampicin covered polyesters and cryopreserved allografts may be more suitable in the presence of prosthesis-duodenal fistula, but, ultimately, choice depends on local protocols, patient condition and preference of the surgeon.13 Instead of using prosthetic grafts, autologous conduits have proven to be a superior alternative, more resistant to infection and with longer durability. For aortoiliac reconstruction, femoral vein is usually the preferred autologous option since it can work with different possible configurations, like the neoartoiliac system (NAIS) in a pantaloon configuration (Figure 2). Due to the use of autogenous tissue, rates of re-infection are lower and long-term antibiotic therapy is usually unnecessary.16 A summary of the different graft options is described in the Table.13,15,16

Figure 2 Femoral vein graft created neoaorta (NAIS) procedure with aorto-bi-iliac reconstruction with two femoral veins in a pantaloon configuration after an EVAR explantation.
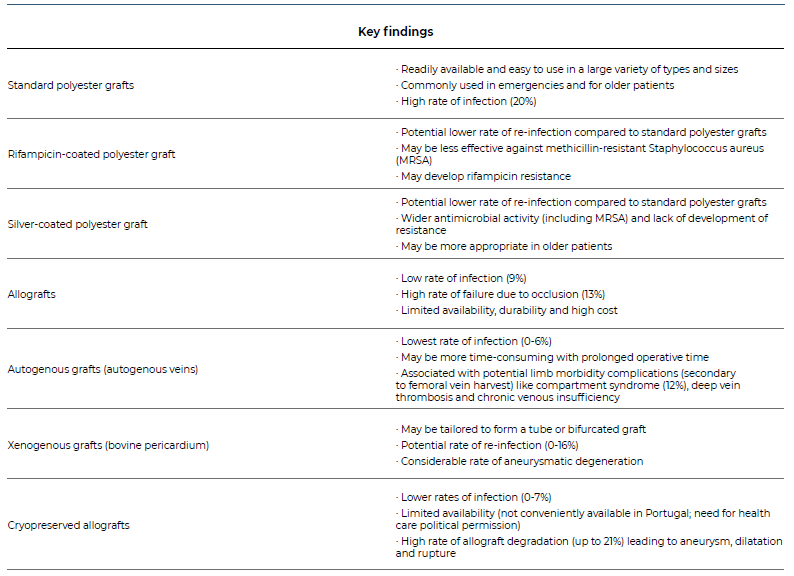
Table 1. Summary on the different graft options for in-situ reconstruction after secondary aorto-enteric fistula.13,15
Advantages for in-situ reconstruction include absence of aortic stump complications like blowout or ascending aortic thrombosis, and association with better primary patency with lower reintervention rates for graft occlusion and consequent decreased amputation rates.3,5,17 Despite the lack of guidelines concerning this topic, it is recommended that it is avoided in case of gross peritoneal contamination with purulent material or in the case of high surgical or haemodynamically unstable patients.9
The prevalence of choice of each technique is variable. A review by Koda et al. that included 32 patients reports AAA resection and stent graft excision in 23 patients (72%), followed by an extra-anatomical bypass in 14 (61%) and in-situ replacement in 9 (39%).7 The other patients were either treated by primary repair of the aortic defect with a pericardium patch (1 case), antibiotic therapy alone (3 cases), were not submitted to any kind of treatment (2 cases) or their treatment is unknown (3 cases). On the other hand, the multicentre study by Kahlberg et al., with 32 cases of post-EVAR AEF, reports surgical treatment with complete graft explantation in 27 cases (84%), followed by extra-anatomical revascularization in 13 cases (48%) and in-situ replacement in 14 (52%).3
Bowel is most often repaired primarily by suture of the defect, but enteric segmental resection may also be necessary when the defect is large.7 Graft or defect coverage with autologous tissue (usually omentum but other options include muscle, fascia or retroperitoneal tissue) is also frequently performed13 This technique may also be used in case of extra-anatomical revascularization for protection of the aortic stump and minimisation of blowout risk.7
Finally, a conservative approach with medical treatment and stent graft preservation may be the only option in patients who are at a prohibitive high risk for major surgery. If possible, drainage of the infection without stent graft removal should be considered.13 However, a conservative approach is associated a 100% mortality rate at 1 year of follow-up since the patient is in a constant condition of infection due to contact of the prosthesis and intestinal content.18 Antibiotic therapy may temporarily control infection, but without surgery there will not be a complete eradication of the source.
Antibiotic therapy is a mainstay of treatment in both operated and conservatively managed patients, and it should be preferably directed by culture sensitivity.3,9 There are no guidelines regarding the optimal duration of treatment in AEF after EVAR, so timing is highly variable in literature reports. A minimum of 4 weeks is recommended, with some authors advocating antibiotic therapy for several months to a year, or until clinical and laboratory parameters of infection have normalised.3,9,13 In patients where complete graft explantation was not possible, cultures were positive for a highly virulent organism or a conservative approach was chosen, lifelong antibiotics may be recommended.3,13Considering the complexity of interpreting microbiological test results or choice of empiric therapy before those are available, a multidisciplinary management is recommended, with a team that includes vascular surgeons, infectious diseases specialists, microbiologists, radiologists and gastroenterology specialists.13
Prognosis
AEF following EVAR is associated with a high mortality rate, even after adequate surgical treatment and independently of the chosen technique. Mortality rates were as high as 100% in some smaller series6, but more recent literature shows better outcomes (17-37%).3,7 High morbidity and mortality causes include the technical complexity of the surgery, anaemia and sepsis at presentation and usual poor medical condition in most patients with this complication.3,9 It is also worth noting that death in patients with post EVAR AEF may also be related to the common history of atherosclerotic disease (myocardial infarction and stroke) and not necessarily because of the AEF itself.3
Conclusion
Secondary AEF is a rare complication after EVAR that is associated with a high morbidity and mortality. Clinical presentation is usually non-specific, hampering the diagnosis, which should be swift to initiate proper control of the infection. Treatment requires graft excision, arterial reconstruction, bowel repair and prolonged antibiotic therapy. The goal of this narrative review was to provide recent evidence on this complication, which may be on the rise as endovascular procedures are becoming ever more common. Literature is still limited regarding this topic, creating a need for more multicentric studies including a higher number of patients in order to provide more insight on how to identify and treat this lethal complication.















