Introduction
After the first US Food and Drug Administration-approved endovascular abdominal aortic repair (EVAR) in 1999 and thoracic endovascular aortic repair in 2005, endovascular repair of TAAA aortic aneurysms (TAAAs) and abdominal aortic aneurysms (AAAs) has gained popularity. It is now considered a standard of care. Not all anatomy and clinical scenarios, however, can be managed within the instructions for use of current commercially approved devices.1 Next-generation EVAR technology incorporates aortic side branch vessels using fenestrations and side arm branch cuffs. Although dedicated, manufactured fenestrated/branched endovascular aortic repair (F/BEVAR) stent-grafts for more complex TAAA aortic pathologies remain in the investigational phases in the United States, with access limited to a few centers with Investigational Device Exemption (IDE) protocols, physician-modified endografts (PMEGs) provide an alternative strategy whereby fenestrations and branches can be created by the treating physicians using off-the-shelf endovascular components.1-3 In this article, we review the indications, techniques, and outcomes of PMEGs for complex AAAs and TAAAs in our center.
The clinical files of all patients undergoing PMEGs were consulted, and demographic data as surgery outcomes were collected. Technical success was defined as creating the intended number of fenestrations, target vessel catheterization, and vessel patency. Procedural success was defined as technical success with exclusion of the aneurysm without direct endoleaks in the final angiography. Thirty-day complications and mortality were also evaluated. Technical and procedural success were assessed, as well as morbidity and mortality.
Case reports
CASE 1:
Male 85-year-old with a relevant past medical history of myocardial infarction, dual-chamber rate-modulated pacing, high blood pressure, and chronic kidney disease presented at the emergency room with abdominal pain with one month of evolution.
At clinical examination, the patient was normotensive, normocardic, and had an epigastric pulsatile mass with light abdominal pain at profound abdominal palpation.
Laboratory results showed a stable hemoglobin (Hb) of 12.8 g/dL, urea 120 mg/dL, creatinine 2.18 mg/dL, and estimated glomerular filtration rate (eGFR) of 27 mL/min/1.73.
Computed Tomography Angiography (CTA) showed an 8 cm pararenal AAA (Figure 1-A) with no evidence of rupture. It was characterized by an inadequate proximal landing zone for standard EVAR, as the main left renal artery (LRA) emerged directly from the aneurysm (Figure 1-B).
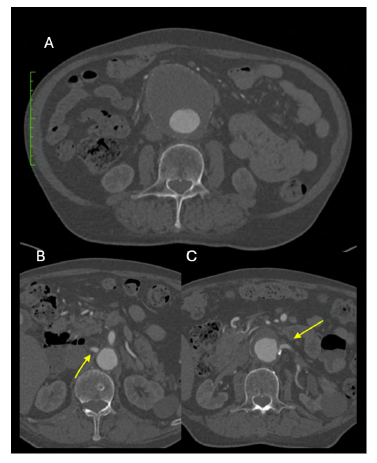
Figure 1 Pre-operative computed tomography angiography (case 1). A - 8 cm Left Juxtarenal AAA. B - the main left renal artery emerged directly from the aneurysm. C - the right renal artery is 1cm proximal from the aneurysm.
Given the patient’s age, frailty, and complex anatomy, an endovascular approach was favored. Assuming the aneurysm was symptomatic, customized options were discharged (due to time constraints). As the right renal artery (RRA) was 1cm distant from the aneurysm (Figure 1-C ) and had an acceptable diameter to allow an adequate sealing zone, exclusion of the aneurysm was done using an Endurant IIs device, with back-table fenestration and stent-graft placement into the left renal artery.
The modification design was first planned using a center lumen line (CLL) reconstruction, with measurement of the diameter of the RRA (6 mm). It was assessed that it emerged from the aorta at 1:00.
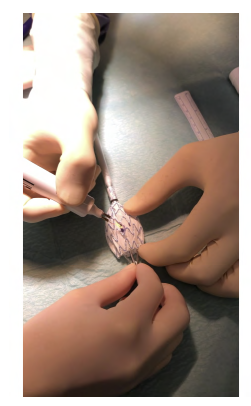
In a back table, we unsheathed the initial portion of the endoprosthesis Medtronic® Endurant Iis. We marked the right renal fenestra with a dermographic pen at the intended 1 pm position, and a 6mm diameter fenestration was created with thermocautery. A portion of a “snare” loop was sutured around the fenestration with a 6/0 proline for radiopaque marking of the fenestrae (Figure 2). Finally, two “reducing ties” were placed below and one above the fenestration.
The endograft was uneventfully resheated (Figure 3), and the EVAR procedure was performed (mainbody 36 mm placed via left femoral artery). The left renal artery was successfully catheterized with a 7Fr sheath, and an Advanta 7x32 mm stent was subsequently deployed and flared with a 10 mm balloon (Figure 4) after the main endoprosthesis ballooning in the fenestrated area to release the reduction ties (Figure 5).
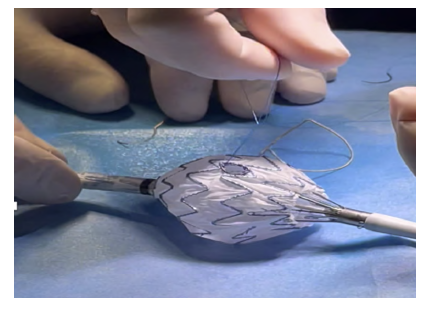
Figure 2 Intra-operative image of graft modification (case 1). Use of a "snare" loop for radiopaque marking of the fenestrae, suture with 6/0 prolene.
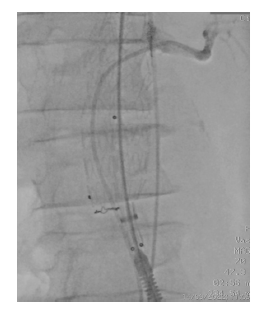
The control angiography is presented in Figure 6, demonstrating good stent graft positioning, visceral and renal artery patency, and no endoleaks. The patient was discharged asymptomatic one week after surgery. A 30-day control CTA showed total AAA exclusion, LRA patency, and no endoleaks (Figure 5). No decline in renal function was also found.
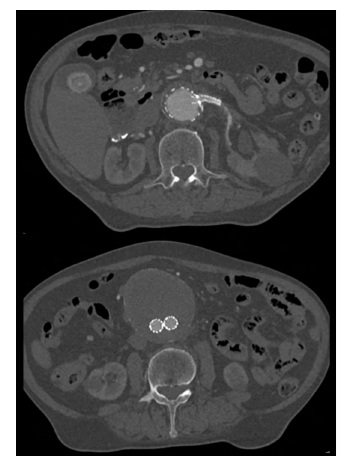
Figure 5 Post-operative computed tomography angiography (case 1). Patency of the left renal fenestration (top) and aneurysm exclusion (bottom)
CASE 2:
A 62-year-old male with a relevant past medical history notable for chronic hepatic disease with portal hypertension (esophageal and gastric varices with multiple episodes of upper gastrointestinal bleeding and various interventions, gastropathy and hypersplenism with pancytopenia), hypertension, and diabetes presented at the emergency room with abdominal pain with lumbar irradiation, with one month of evolution but worsening in the last three days.
At clinical examination, the patient was hypertensive, normocardic, and had an epigastric pulsatile mass, which was not painful on abdominal palpation.
A CTA was performed, which revealed a type V TAAA aortic aneurysm with a maximum diameter of 53mm and morphology suggestive of an infectious etiology and contained rupture (Figure 6).
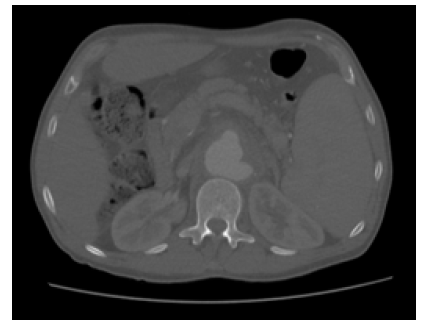
Figure 6 Pre-operative computed tomography (case 2). Axial image revealing a type V thoracoabdominal aortic aneurysm a maximum diameter of 53mm with mycotic morphology and contained rupture
The descending aorta had a diameter of 25 mm, while the visceral and infrarenal aorta were smaller (17 and 16 mm, respectively). Also noteworthy, the celiac artery (CA) was occluded (Figure 7). Given the acute presentation of a symptomatic aneurysm, with suspected contained rupture, urgent surgery was indicated. Attending to the patient's medical history, we considered him a high-risk patient unfit for open surgery. Given the urgent nature of the situation, custom-made stent grafts were not possible. The aneurysm presented unfavorable anatomical characteristics for a Cook® T-Branch device or parallel/chimney stent graft repair. Thus, a PMEG was prepared after precise planning using a CLL (Figure 8). Fenestrations were placed for the superior mesenteric artery (SMA), LRA, and RRA in Medtronic® Valiant 32x32x157 mm stent graft.
After unsheathing the proximal portion of the device, fenestration positions were measured and marked (at 90 mm and 102 mm after the start of the endoprosthesis: at 3:00 the LRA; 9:00 the RRA and 12:15 the SMA, and 6mm and 8mm fenestrations were created for the renal arteries and SMA (Figure 9) with the previously described technique (Figure 10).
Two "reducing ties" were placed below and above the fenestrations. The stent graft was then positioned from left femoral access and successfully deployed, followed by renal and SMA catheterization, 7 Fr sheath, and Advanta V12 stent positioning (LRA and RRA - 6x22mm, SMA - 6x32mm), stent graft ballooning (releasing the reducing ties), and finally target artery stent deployment and flaring (10mm balloon). The control angiography showed adequate stent graft and visceral artery stent deployment, with no endoleak (Figure 11). The patient was discharged asymptomatic one week after surgery, and seven days after, the control CTA showed complete aneurysm exclusion with no visible endoleaks and SMA and renal artery patency (Figure 12).
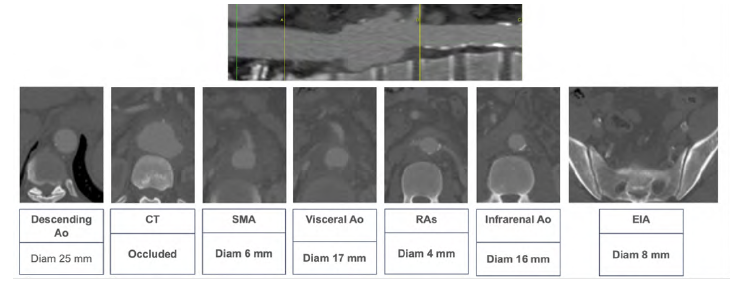
Figure 7 Pre-operative computed tomography - anatomical details (case 2). Above, a center-lumen line reconstruction is shown. Below, sequential axial images of the aorta at different levels highlight the most relevant anatomical features.
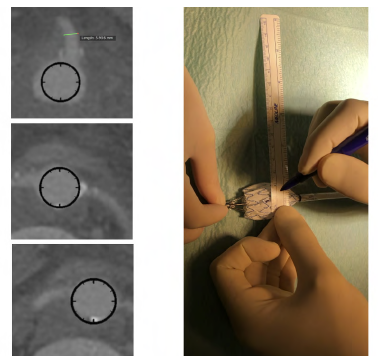
Figure 8 Planning and marking of fenestrations (case 2). After precise planning using a center-lumen line, we proceeded to the construction of fenestrae for the superior mesenteric artery, left renal artery, and right renal artery using a Valiant 32x32x157 stent graft. We then marked the location of the fenestrae with a ruler (9cm and 10.2 cm after the start of the endoprosthesis at 3 a.m., 9 am, and 12:15 a.m.).
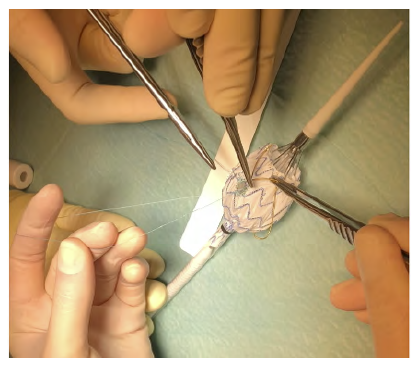
Figure 10 On-table stent graft modification (case 2). Reinforcement and radiopaque marking of the fenestration using a snare loop and 6/0 prolene.
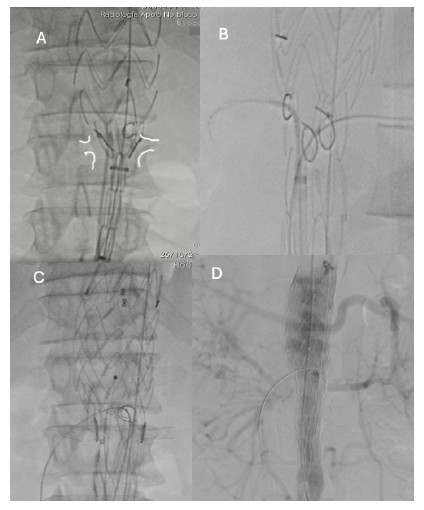
Figure 11 Intra-operative radioscopy images (case 2). A - PMEGs’deployment after angiography after marking of both renal arteries; B - Bridging stent placement in renal arteries and C - superior mesenteric artery; D - Control angiography showed adequate graft positioning, visceral and renal vessel patency and no endoleak.
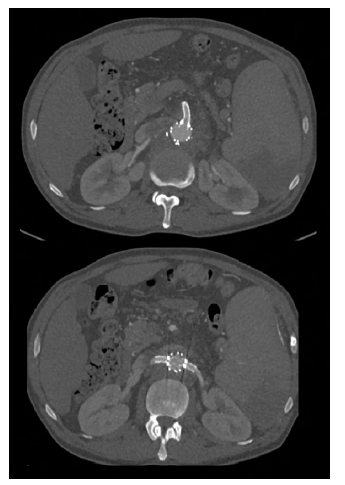
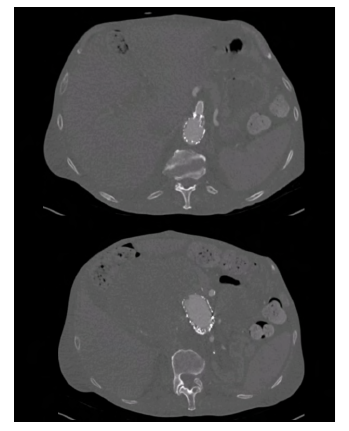
Figure 12 Post-operative computed tomography angiography (case 2). Axial slices showing the patency of the superior mesenteric artery (top) and renal arteries (bottom).
Case 3:
An 83-year-old male with a relevant medical background for paroxysmal atrial fibrillation, hypertension, and dyslipidemia had undergone standard EVAR 4 years prior to presentation for a 66 mm AAA. He presented to the emergency room with abdominal pain with lumbar irradiation, which progressively worsened over the previous three days. A CTA showed that the AAA sac had grown to 11.3cm due to a type 1A endoleak. The patient underwent urgent repair with an additional infrarenal cuff insertion and endoanchoring (Medtronic Heli-Fx®). The final angiography showed that the type 1A endoleak had been resolved, but inadvertent bilateral renal artery coverage had been performed. Several endovascular salvage attempts failed to rescue the renal arteries, leaving the patient in chronic renal replacement therapy (a brachial-cephalic arteriovenous fistula was subsequently constructed).
After nine months following the secondary aortic procedure, he presented once more to the emergency department with abdominal pain with lumbar irradiation. A new CTA was performed, which showed further growth of the aneurysm sac, which now measured 16 cm in diameter, and the presence of a type 1B endoleak. An urgent endovascular repair was performed with parallel graft deployment to the left hypogastric and external iliac arteries. The postoperative CTA revealed a type 2 endoleak originating from the inferior mesenteric artery (IMA), which was further corroborated by contrast-enhanced duplex ultrasound. Open IMA ligation was performed through a median suprapubic laparotomy. No manipulation of the AAA sac was performed during the procedure.
On a control, CTA, an undetermined endoleak located close to the most cephalic and anterior portion of the aneurysmal sac was found (possible type 1A vs. type 2 endoleak). Also, additional AAA sac growth was observed, with the AAA sac now measuring 19.7 cm in diameter (Figure 13).
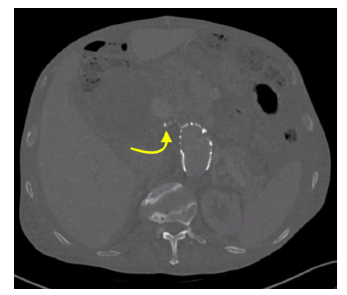
Figure 13 Pre-operative computed tomography angiography (case 3). A proximal endoleak of undetermined origin is noted (arrow)
A type 1A was then assumed, and an additional aortic cuff modified with a single fenestration for the SMA was performed (the CA was chronically occluded). The control CTA showed no aneurysmal sac growth or evidence of the preoperative endoleak (Figure 14). At the 30-day follow-up, no further interventions or adverse events had occurred.
Discussion
The PMEG technique is an off-label procedure that customizes standard stent grafts to include fenestrations. Thus, it allows the treatment of patients with complex aortic anatomy by including the visceral and renal vessels.
PMEGs and parallel grafting (PG) are important techniques for endovascular repair of complex aortic aneurysms using off-the-shelf devices. However, more data should be available regarding these techniques' relative efficacy and outcomes in TAAA aneurysms. Some large retrospective studies show that survival after endovascular TAAA repair is better with the use of PMEG compared with PG.4
All treatment options for complex AAAs and TAAAs should be considered carefully when planning a repair, with PMEGs being reserved for those cases unsuitable for other repair alternatives when performed at centers with the volume and expertise to execute the procedure with high technical success and low morbidity and mortality.1,5
In our present study, between December 2020 and December 2022 (24 months), only three patients underwent PMEGs, with limited follow-up. Regarding to the type of aneurysm, one case was a juxtarenal aortic aneurysm, another a type V TAAA and one case of persisting proximal endoleak with progressive sac enlargement. All patients were symptomatic, with one case presenting as a contained rupture. The mean number of target arteries incorporated was 1.7 per patient. As reported, our center presented 100% technical and procedural success, with no mortality or morbidity at the 30-day follow-up. Despite being a small series, we had excellent results with this technique concerning the current literature.1-5 We can conclude that modification planning is crucial to successfully implementing a PMEG.
Several novel approaches to endovascular management of aortic aneurysms are being explored, and several groups have described back-table fenestration. This approach relies heavily on precise preoperative imaging, exact measurements, and device deployment. Larger series with long-term follow-up will be necessary to enhance our understanding of appropriate patient selection for this technique.