Introduction
The management of patients with asymptomatic carotid stenosis is still a matter of debate. European Society for Vascular Surgery (ESVS) guidelines published in 2023 advise that carotid interventions should be reserved for a small subset of patients whose imaging/clinical criteria make them at higher risk of stroke on medical therapy.1
In recent years, evidence on the relation between asymptomatic carotid stenosis and the development of cognitive impairment has been published.2,3
Some potential mechanisms were explored and included silent cerebral infarction, silent embolization, involvement in the pathophysiology of lacunar infarction or white matter hyperintensities, or hemodynamic changes.4,5 Several questions arose on the impact of carotid interventions in the prevention and/or reversion of cognitive decline in this subset of patients.
This study aimed to examine the impact of carotid artery stenting (CAS) on various domains of cognitive function in patients with asymptomatic carotid artery stenosis.
Methods
This review aimed to identify studies that evaluated cognitive function in patients with asymptomatic carotid artery stenosis that were submitted to CAS and published up to February 2023. PubMed and Scopus databases were searched using the following query “(cognitive function OR cognitive impairment OR cognitive changes OR cognitive performance OR cognitive) AND carotid AND (endarterectomy OR stenting)”. All original studies assessing both pre- and post-operative cognitive functions in patients with asymptomatic carotid stenosis undergoing CAS were considered eligible for inclusion. Only publications in English were considered. There were no restrictions concerning age, gender, or number of participants. All editorials, letters, case reports, review articles, systematic review, meta-analysis, and animal studies were excluded. Two researchers independently screened titles and abstracts individually for eligibility. Disagreements were solved by discussion between both authors.
Results
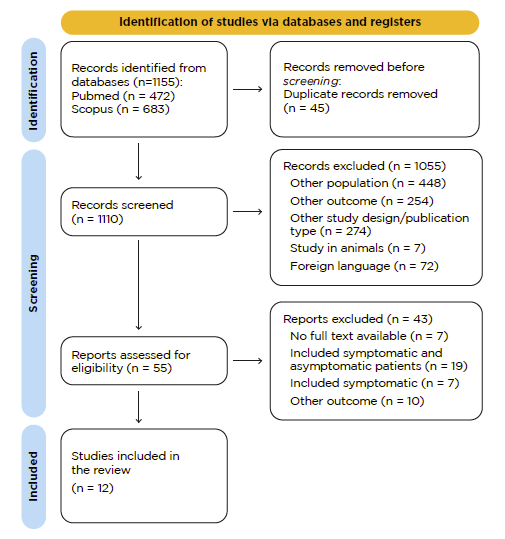
After screening, 12 studies were eligible for our review, including 273 CAS in asymptomatic patients, Figure 1. Table 1 presents the main characteristics of the included studies.
Table 1 Main characteristics of studies evaluating cognitive function after asymptomatic carotid artery stenting, included in the literature review.
| Study (Author, year) | Study Design | Number of CAS | Mean Age (years) | Preoperative assessment | Postoperative assessment | Global Congition | Executive Function | Language Ability | Memory | Attention/ psychomotor speed | Functional Ability |
| Tiemann, 2009 | cohort study, prospective | 22 | 67.8 | 1-3 days before | After ≥6 weeks | N.R. | N.R. | German vocabulary test | List learning test | WAIS; Number-connecting-test | N.R. |
| Feliziani, 2009 | cohort study, prospective | 24 | 75.6 ± 5.7 | 1 day before | At 3 and at 12 months after | MMSE | TMT A; TMT B; COWA | N.R. | BSR; Rey-IR; Rey-DR; CNT | N.R. | ADL; IADL |
| Lal, 2011 | cohort study, prospective | 21 | N.R. | 1-3 days before | Between 4-6 months after | N.R. | TMT A; TMT B | BNT; Controlled Oral Word | Working Memory; HVLR | Processing Speed Index | N.R. |
| Mendiz, 2012 | cohort study, prospective | 20 | 70.9 ± 7.5 | ≤48 hours before | At 3 months after | MMSE; ACE-R; RAVLT | backward digit span task; phonological fluency; TMT B; modified version of the Wisconsin Card Sorting Test; INECO Frontal Screening; TMT A | BNT | ROCF | WAIS III | N.R. |
| Cappocia, 2012 | cohort study, prospective | 28 | 71.7 ± 7.2 | ≤24 hours before | At 6 and at 12 months after | MMSE | N.R. | N.R. | N.R. | N.R. | N.R. |
| Ortega, 2014 | cohort study, prospective | 25 | 74 | ≤24 hours before | At 6 months after | N.R. | Token test; Stroop color test | BNT; COWAT; California verbal learning test | N.R. | WAIS III | N.R. |
| Kougias, 2015 | RCT, prospective | 29 | N.R. | ≤2 weeks before surgery | At 6 weeks and at 6 months after | RAVLT | Stroop Color test; Stroop Word; TMT A | FAS | BMVT-R | WAIS-IV | N.R. |
| Wang, 2017 | cohort study, prospective | 16 | 66.8 ± 5.8 | 7 days before | At 3 months after | MMSE; MoCA; RAVLT | N.R. | N.R. | Verbal Memory Test | Digit Symbol Test | N.R. |
| Turowicz, 2021 | cohort study, prospective | 20 | N.R. | N.R. | N.R. | MoCA; CANTAB | N.R. | N.R. | N.R. | N.R. | N.R. |
| Lin, 2022 | cohort study, prospective | 23 | 67.8 | N.R. | N.R. | MMSE; RAVLT | TMT A; TMT B; Stroop Color test | N.R. | N.R. | N.R. | N.R. |
| Ning, 2022 | cohort study, prospective | 25 | 70.48 ± 4.35 | preoperatively | At 3, at 6 and at 12 months after | MoCa; RAVLT | N.R. | N.R. | N.R. | N.R. | N.R. |
| Fischer, 2022 | cohort study, prospective | 20 | 65.6 ± 8.9 | ≤10 days before | Between 6-10 weeks after | MMSE | Stroop-Test; TMT A; TMT B | N.R. | N.R. | N.R. | N.R. |
N.R. - No reference; CAS - Carotid artery stenting; MMSE - Mini-Mental State Examination; MoCA - Montreal cognitive assessment; RAVLT - Rey Auditory Verbal Learning Test; TMT - Trail Making Test; BNT - Boston Naming Test; BSR - Babcock Story Recall; HVLR - Hopkins Verbal Learning Test; ROCF - Rey-Osterrieth Complex Figure; WAIS - Wechsler Adult Intelligence Scale; IADL - Instrumental Activities of Daily Living; COWAT - Controlled Oral Word Association Test; CANTAB - Cambridge Neuropsychological Testing Automated Battery; BMVT-R - Brief Visuospatial Memory Test-Revised; ACE-R - Addenbrooke's Cognitive Examination Revised; CNT - Computorized neurocognitive test
Eleven articles were prospective cohort studies, and one article was a randomized controlled trial. The mean age of included patients was 70+/-13.4 years, and approximately 63% were male (n=170 male patients), although two studies did not mention gender. Only seven articles reported the type of anesthesia, and locoregional techniques were used in all reported cases. Regarding cerebral protection, in eight studies, a distal filter was used; one study used flow reversal; in another, no protection device was used; three studies did not mention if any technique for cerebral protection was used during the procedure.
In the included studies, the effect of CAS was evaluated in different cognitive domains with specific tests/assessments: global cognition (using Mini-Mental State Examination (MMSE), MoCA (Montreal cognitive assessment) and Rey Auditory Verbal Learning Test (RAVLT)), executive function (using Trail Making Test (TMT) A or Color Trails Test (CTT) A and TMT B or CTT B), language ability (using Boston Naming Test (BNT)), memory (using Verbal Fluency (VF), Babcock Story Recall (BSR), Hopkins Verbal Learning Test (HVLR), Rey-Osterrieth Complex Figure (ROCF)), attention/psychomotor speed (using Digital Symbol - Wechsler Adult Intelligence Scale (DS-WAIS)) and functional ability (using National Institutes of Health Stroke Scale (NIHSS) and Instrumental Activities of Daily Living (IADL)).6,7
The MMSE is frequently selected as a test due to its simplicity, reliability, and large clinical application. It is sensitive to attention, repetition, and language but not to abstract thinking, judgment, problem-solving, and prediction. The tests used for evaluating each cognitive domain in the included articles are presented in Table 1.
Discussion
Cognitive function and carotid stenosis
Cognitive impairment significantly affects patients’ functional status, social interactions and emotional well-being, and it is increasingly recognized as an important outcome. The contribution of carotid artery stenosis to cognitive impairment has traditionally been considered solely related to the occurrence of cerebral ischemic lesions.8 The Tromsø Study compared patients with asymptomatic carotid stenosis and asymptomatic patients without carotid stenosis (control group), and found that patients with carotid stenosis had a poorer neuropsychological and cognitive tests results; also, the presence of carotid stenosis was a risk factor for cognitive impairment, even in patients without vascular lesions in brain MRI.9 Thus, the Tromsø study showed that carotid stenosis could be considered as an independent risk factor for cognitive impairment. Furthermore, an association between the severity of the stenosis and the extent of cognitive deterioration was established in the Cardiovascular Health Study.10
The negative impact of carotid stenosis on cognitive efficiency has several mechanisms. Carotid stenosis causes a reduction in cerebral perfusion pressure which can progress to a chronic hypoperfusion state (perfusion-weighted MRI can evaluate that).10 Hypoperfusion, usually counterbalanced by collateral vessel systems, can be perpetuated by blood flow derangement, contributing to worsening cerebral functions, including cognitive activities.11,12 The microembolization by unstable or ulcerated plaques can also contribute to lacunar infarcts. According to Brottet et al., 15 to 19% of patients with carotid stenosis have a documented brain microembolization. Microembolization could contribute to cognitive impairment by inducing ischemic damage and micro-circle derangement.13
Cognitive impairment in patients with carotid stenosis is the final result of the complex interaction between different elements and mechanisms that traditionally include hypoperfusion and silent infarctions.14
Carotid artery stenting and Cognitive Impairment
Eliminating a potential embolic source by carotid revascularization may be expected to improve neurocognition uniformly. However, the effect of CEA and CAS on neurocognitive functions in patients with carotid artery stenosis remains controversial. In 2008, a systematic review of the impact of CAS and CEA on cognitive function showed that neither procedure could affect cognition.15 Years later, in 2014, another systematic review compared the impact of CEA vs. CAS in postoperative cognitive function. Of 13 included studies, most did not show significant differences in overall cognitive function nor between CEA and CAS.16
Paraskevas et al. showed that patients with asymptomatic carotid stenosis with impaired cerebral vascular reserve (CVR) were significantly more likely to have cognitive impairment. On the other hand, patients with severe asymptomatic carotid stenosis but normal CVR can have cognitive scores similar to controls. Thus, impaired CVR could represent a high-risk group for cognitive impairment.5
According to our results, studies on cognitive performance after CAS exhibit wide variations in the timing of assessment, specific tests performed, and use of cerebral protection devices. Additionally, the study populations are small, and several possible confounding factors such as age, contralateral carotid or vertebral artery disease, severity of carotid stenosis, and others can impact results. A limitation in the analysis is evident, and several attempts to assess the effect of CAS on cognitive function have yielded conflicting conclusions. Consequently, the debate over the optimal management of asymptomatic carotid patients is ongoing.
Currently, the consideration of carotid revascularization vs. best medical therapy in asymptomatic carotid artery stenosis patients is primarily based on the risk of stroke/TIA. If recent trials fail to demonstrate a difference in the incidence of these adverse events, the superiority of a procedure could be based on other relevant patient-centered outcomes, such as neurocognitive outcomes. However, high-quality data must still be provided to guide the management approach.
Future Directions and Controversies
There are still some unclear data and conflicting results in the current literature. Several cognitive assessment tests are employed, and there is no consensus on the tests used in different studies. Ideal cognitive assessment tests in patients with asymptomatic carotid stenosis should be simple, not require a neuropsychologist, and reproducible while avoiding significant practice or learning effects. Standardized cognitive assessment tests, along with studies involving larger sample sizes, could enhance statistical power, facilitate comparisons across studies, and improve the generalizability and reliability of findings. Furthermore, there needs to be more agreement on the timing of the pre and postoperative cognitive assessments in published studies. Ideally, neurocognitive assessment should be conducted preoperatively for baseline evaluation, as well as postoperatively (within 30 days), and at least six months to one year after the procedure. Conducting studies with extended follow-up periods could also be beneficial in providing a better understanding of the long-term effects of CAS on cognitive function. In addition, it is crucial to identify potential biomarkers and genetic factors that may help to predict patients’ responses to treatment and identify patients who are more likely to benefit from CAS regarding cognitive outcomes. Exploring advanced neuroimaging techniques, such as functional MRI or positron emission tomography (PET), could add valuable information on the possible mechanisms and brain regions involved in cognitive improvement following CAS.
Conclusion
Asymptomatic carotid stenosis has been linked to neurocognitive decline. The effect of CAS on cognitive function is still a matter of debate. The heterogeneity and inconsistency of data in available studies limit conclusions. Further studies with standardized cognitive assessment tests, larger sample sizes, and consensual assessment timetables are needed to understand better the impact of CAS in asymptomatic carotid stenosis patients. Improving cognitive functions with CAS can be considered a relevant patient-centered outcome, especially in higher-risk subgroups, such as patients with impaired CVR.