Introduction
Aorto-bifemoral bypass (ABF) remains the gold standard treatment for complex aortoiliac occlusive disease. Although ABF can be performed safely in lower-risk patients, some present with severe pararenal aortic neck calcification and thrombus that precludes the ability to construct a juxta-renal anastomosis safely. Similarly, certain patients have severe native para-renal and mid-visceral aortic atherosclerotic disease that, due to clinical symptoms and anatomic proximity, mandates concurrent reno-mesenteric arterial reconstruction if in-line aortic repair is attempted. In these scenarios, an alternative to primary or “redo” ABF is the thoraco-bifemoral (TBF) bypass.1,2
Case report
We present the case of a 57-year-old man with a personal history of hypertension and smoking. The patient presented to the emergency department due to flank pain and anuria, with two days of evolution, requiring urgent dialysis.
A computed tomography angiography (angio-CT) was performed, revealing thrombosis of the abdominal aorta, with a chronic appearance in the infrarenal segment and acute in the suprarenal segment; permeability of superior mesenteric artery (SMA) and celiac trunk (CT); but involved with fresh thrombus in the posterior aspect of the aorta; distal patency of the right renal artery due to an adrenal collateral; and chronic occlusion of the left renal artery, with left kidney atrophy, Figure 1.
In the face of acute juxta-renal aortic thrombosis with acute kidney ischemia, the patient was proposed for emergent revascularization and treated with a parallel graft technique using covered stents (Advanta V12) to revascularize the right renal artery and ensure patency of the coeliac trunk and superior mesenteric artery. Accordingly, the celiac trunk received a 9x38mm stent, the SMA 8x59mm stent, and finally for the RRA a 7x59mm extended proximally with an 8x38mm covered stent. Simultaneous balloon molding was performed with 10x40mm balloons in each of the three revascularized arteries (Figure 2), and the completion of the angiography demonstrated good permeability. The patient was discharged one week later with complete recovery of renal function.
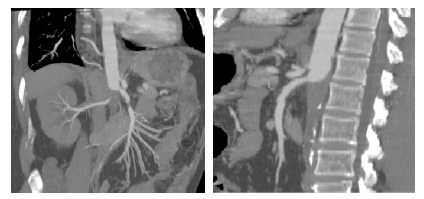
Figure 1 Pre-operative computed tomography angiography (coronal and sagittal multiple-intensity projections)
Thrombosis of the abdominal aorta can be noted, with a chronic appearance in the infrarenal segment and acute in the suprarenal segment. Both renal arteries are occluded, and there is thrombus extension to the level of the mesenteric vessels.
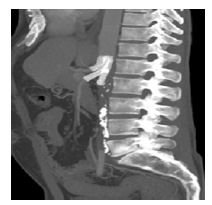
Figure 2 Computed tomography angiography after endovascular visceral revascularization (sagittal multiple intensity projection)
Visceral reconstruction with parallel stent grafts to the coeliac trunk, superior mesenteric artery and right renal artery
During follow-up, the patient presented with complaints of disabling intermittent claudication, with progressive worsening up to a walking-free distance of only 5 meters. This was severely disabling and incompatible with his profession. He had no rest pain. After cardio-pulmonary assessment with resting transthoracic echocardiogram and respiratory function tests, we decided to perform a TBF bypass, preserving the prior endovascular reconstruction.
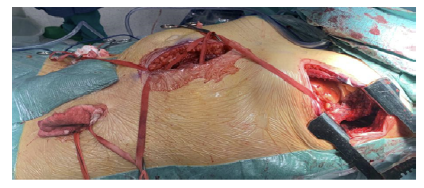
The patient was positioned in a right lateral decubitus position using a bean bag, with the bed flexed between the anterior superior iliac crest and caudal margin of the ribs, Figure 3. The hips were left flat to facilitate femoral artery exposure, and the upper torso was rotated 30 degrees with the left shoulder elevated and immobilized on an armrest. Oro-tracheal intubation was performed with a double-lumen tube and pulmonary exclusion during the proximal anastomosis.
A left thoracotomy was performed through the 9th rib space. The inferior pulmonary ligament was transected, and the left lower lobe was retracted to expose the distal descending thoracic aorta. Standard exposure was done for the femoral arteries. The graft was tunneled through a small incision in the muscular portion of the diaphragm, close to the lateral chest wall, and the left limb was further tunneled retroperitoneally to the left femoral artery. The right graft limb was positioned preperitoneally due to the potential for kinking during crossed tunneling from the left groin to the right.
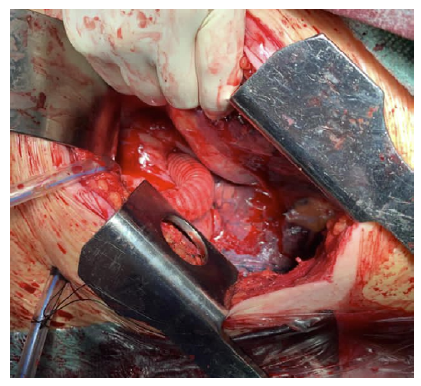
The anastomosis to the descending thoracic aorta was performed end-to-side (Figure 4), with tangential clamping avoiding aortic occlusion. The graft was angled to be directed to the previously created tunnel. Standard end-to-side femoral anastomoses were constructed.
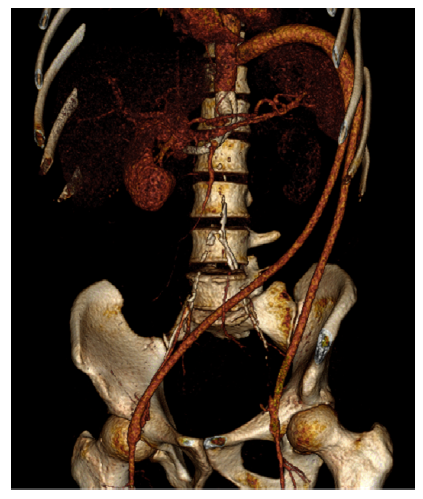
The procedure was uneventful. The patient was discharged eight days after surgery. Postoperative CT angiography revealed visceral aorta branches and TFB patency. (Fig. 5)
Clinical and duplex ultrasound surveillance of the TBF bypass and previous visceral revascularizations was performed one month after intervention, at 6-month intervals for the first two years, and is scheduled to be performed annually after that. The patient remained asymptomatic with palpable pedal pulses at two years' follow-up. An angio-CT is planned five years after surgery to evaluate TFB patency, assess proximal anastomotic integrity, and exclude the development of an anastomotic pseudoaneurysm.
Discussion
The ABF bypass has long been established as the gold standard treatment for severe aorto-iliac disease due to its well-documented applicability to most anatomic patterns of disease, low morbidity and mortality, and good overall durability. Specifically, elective major perioperative morbidity rates of 10 to 30% and mortality outcomes of 1-4% are reported.1,2 Moreover, the excellent hemodynamic impact of AFB bypass has resulted in some of the most durable outcomes for any vascular operation for peripheral arterial obstructive disease, with primary limb patency rates of 76-95% at five years and 75-85% at ten years.1 These outcomes represent the benchmark that all other interventions for complex aorto-iliac disease should be compared.
Despite having superior long-term outcomes for severe aorto-iliac disease compared to other endovascular and/or hybrid (e.g., iliofemoral endarterectomy with retrograde iliac angioplasty/stent placement) strategies, there are specific scenarios where this approach may not be the optimal revascularization choice even in good risk patients. In particular, these situations include patients presenting with TASC II-D lesions and severe juxta-renal/supra-renal aortic calcification or failed AFB bypass with marginal residual native juxta-renal aorta for reconstruction. Another high-risk group of patients includes complex aorto-iliac disease and concurrent renal and/or mesenteric atherosclerotic disease.1,2 In the presented case, the patient had a chronic infrarenal aortic occlusion (without lower limb-threatening ischemia) and developed acute thrombosis of the pararenal aorta, which extended to the right renal artery, which perfused the single-functioning right kidney (patient with chronic occlusion of the left renal artery with atrophic left kidney).
Upon the initial presentation of this case with renal artery thrombosis and acute renal failure, endovascular intervention with covered renal stenting was preferred to reduce the invasiveness of the procedure and the time of renal ischemia. The use of a parallel graft for SMA and celiac trunk with extension of the renal stent to this level was deemed necessary to reduce the risk of subsequent renal stent thrombosis, avoiding flow turbulence and stasis in the parenal aorta around the single renal stent, and also to preserve the visceral vessels from subsequent occlusion, given the posterior extent of fresh thrombus in the aorta. Regarding the use of the “parallel grafts” technique to protect the visceral arteries, this technique is well established in the complex aortic aneurysms or aortic dissection repair. Still, it has not been previously described in pararenal aortic occlusions, to our knowledge. The alternative of simple endovascular thrombus aspiration could have been tried, but the extent of thrombotic material in that segment was considered high risk for re-occlusion. The open alternative, which would be para-visceral aorta thrombectomy, with supra-celiac clamping and possibly graft interposition in the ABF position, was not considered due to the increased invasiveness and because it is associated with a longer duration of the renal ischemia.
Additionally, as the patient did not present limb-threatening ischemia at the initial presentation, lower limb revascularization was deemed unnecessary at that time.
We consider that surgical revascularizations may be reserved for situations where the chance of renal salvage is deemed substantial (limited ischemia, normal pre-existing kidney function, acute thrombosis of pre-existing chronic stenosis with existing collaterals, etc.) and where endovascular treatment has failed or maybe unfavorable owing to technical or anatomic considerations. Nevertheless, the surgical alternative may have the advantage of not leaving the visceral and right renal arteries dependent on stents. It would allow more hemodynamically favorable perfusion of these organs by maintaining outflow through the graft instead of leaving an aortic stump.
By choosing the TBF bypass in the second intervention to address the patients’ incapacitating claudication, the prior visceral revascularization would be preserved. Since aortic cross-clamping was considered at increased risk of visceral and renal stent thrombosis, the proximal anastomosis was performed using partial (tangential) clamping of the thoracic aorta. Alternatively, a ventral aorta technique might be considered but has been judged more invasive than the TBF performed herein.
A left thoraco-retroperitoneal TBF bypass is a technically demanding operation; however, it can be performed safely with good outcomes for patients with severe aorto-iliac occlusive disease. It may be particularly useful for patients with prior visceral revascularization or requiring concomitant visceral reconstruction. These results support the role of TBF bypass in highly selected patients.