Introduction
Arteriovenous Malformations (AVMs) are rare vascular anomalies characterized by high-velocity and low-resistance anomalous connections between the arterial and venous systems through an aberrant vascular nidus composed of dysplastic vessels resulting from aberrant angiogenesis.1,2 They are congenital and when symptomatic they rarely manifest before adolescence. Infection, trauma, and hormonal changes in puberty and pregnancy are known factors that can trigger the rapid expansion of AVMs, as well a incomplete treatment strategy.1,3,4 The cosmetic appearance, edema, pain, bleeding, tissue ischemia or congestive heart failure are clinical presentations of AVMs which may require intervention. AVMs don’t show spontaneous involution and continue to expand with time due to the fast flow in the arterio-venous connections which make them difficult to eradicate.3,4,5 Depending on the location, size, stage and severity of the symptoms, treatment options vary from conservative management to surgical resection. They can be managed conservatively or with drug therapy, with scleroembolization or surgical resection with prior embolization. Several medications have been tried for the treatment of AVMs targeting different angiogenesis and vascular remodeling pathways but none of them has yet been approved as current therapy.6 Endovascular techniques has been gaining ground as a first-line therapy because of its low morbidity but is also an important and necessary adjunct to resection surgery.7,8 Surgical excision of extensive AVMs or adjacent to vital structures is associated to high morbidity and high recurrence rate due to difficulties to achieve its radical resection, but is still the most effective treatment in small and localized lesions.4,9
Early and correct diagnosis is important for proper management and treatment of the patient. Given the clinical variability of the pathology with diverse symptoms and morphology, complex vessel anatomy as well as unpredictable behavior, the effectiveness and response of treatment is uncertain and therefore there is little consensus on the best approach.
There are only few case reports regarding AVM of abdominal wall. We report a case of a giant arteriovenous malformation of abdominal wall (type IIIb of Yakes Classification) treated with surgical resection after prior attempts of scleroembolization.
Clinical case:
54-year-old woman with known history of osteoarticular pathology and dyspepsia, victim of domestic violence. The patient was referred to the vascular surgery department because she had a mass in the abdomen with years of evolution and had worsened in recent years. A mass was observed in the left side of the abdominal wall during the physical examination. The swelling wasn’t mobile with respect to the underlying soft tissues, had a hard consistency, was warm, slightly pulsating and tenderness to touch at the palpation. Clinically she presented easy fatigue with efforts. In the image exams first performed the mass showed infiltration of the internal and external oblique muscles sparing the transverse muscle. Due to the risk of abdominal wall herniation after excision of the AVM, scleroembolization was considered first-line treatment in this case, and two previous attempts of scleroembolization were made with coil embolization of venous nidus by direct puncture and sclerosis with ethanol of arterial afferents by selective catheterization under pulmonary BP monitoring with regression of the mass and symptoms improvement.
Four years after the last intervention, the patient presented lesion growth, measuring about 20x10cm at physical examination, recurrence and worsening of symptoms with severe interference in the QoL. After imaging reassessment and multidisciplinary discussion with general surgery specialist, she was proposed for complete resection of the AVM (Figure 1).
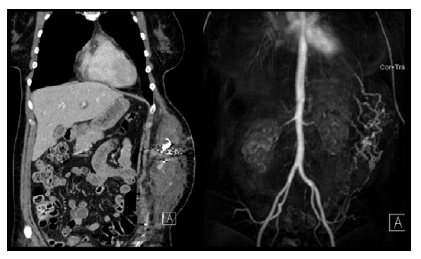
Figure 1 Imaging reassessment prior to surgical resection. Computed tomography angiography and magnetic resonance angiography showed enlargement of soft tissue lesion with multiple dilated tortuous vascular channels involving layers of left abdominal wall.
She was first submitted to scleroembolization with Onyx of identified arterial afferents (internal thoracic, deep circumflex iliac and branch of inferior epigastric arteries) and sclerosis of the lesion nidus by direct puncture with 2% polidocanol in two sessions. In the first one, by selective catheterization and after angiographic confirmation, internal thoracic and deep circumflex iliac arteries were successfully embolized with Onyx. A branch of the inferior epigastric artery was also identified as an afferent artery, but it wasn’t possible to catheterize it despite multiple attempts.
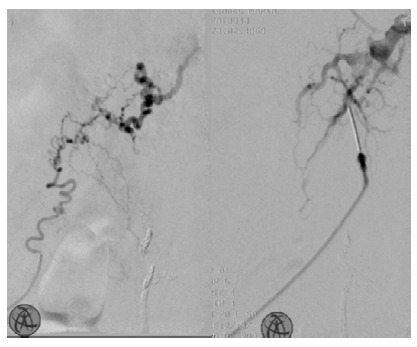
Figure 2 Intra-operative angiography during scleroembolization. On the left: branch of left inferior epigastric artery; On the right: direct puncture of the lesion
We were able to scleroembolize this branch one month later on a second attempt (it was more significant than the previous intervention), after sclerosis of the lesion nidus with 2% polidocanol (Figure 2, left). The final angiography showed partial filling of the lesion, and extra polidocanol was injected using direct puncture (figure 2, right). One month later, she successfully underwent total resection of the AVM with the collaboration of General Surgery under general anesthesia and without perioperative complications (Figure 3).
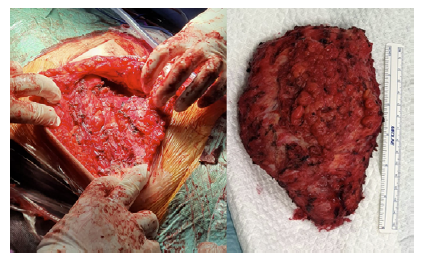
Figure 3 Radical surgical resection of AVM. On the left: lesion in situ; nn the right: surgical specimen
Histological analysis showed that the central area of the specimen was composed of multiple anastomosing blood vessels (arterial and venous), dysmorphic and predominantly large with dilated lumens, interposed by hyalinized fibrosis and foci of hyalinization of the vascular wall. In the surrounding muscle and adipose tissue, a marked dispersed proliferation of small capillaries was identified, with no evidence of atypia or mitosis.
The patient was discharged 5 days after surgery without complications. Eight months after surgery, the patient is asymptomatic without apparent recurrence of the lesion or evidence of abdominal wall herniation.
Discussion
AVMs as their complex anatomy and uncertain behavior remain a diagnostic and therapeutic challenge for all medical disciplines involved in the care of vascular anomalies. AVMs can arise anywhere in the body and therefore have a wide range of clinical manifestations. Clinical signs include dermatologic changes as well as cardiac irregularities in the most severe cases.3,5 The decision for invasive treatment should be made by an interdisciplinary team and is based on individual symptoms and on the impact on the patient’s quality of life, weighed against the risk of complication of the natural course of the disease and the procedure.4,7
Due to the location of the lesion and the risk of abdominal wall herniation in a young patient, in addition to the risk of major bleeding and associated morbidity of surgical resection, endovascular treatment with scleroembolization was the first choice in the above case. However, 4 years later, there was a recurrence of the treatment performed with growth and worsening of symptoms, which reflects probable incomplete scleroembolization in the first attempts. Incomplete treatment and repeated trauma are two described factors that can trigger or accelerate the growth of AVMs. Given major impairment in QoL and impact on daily living activities in a relatively young patient, a prompt and definitive solution was needed. Thus, after discussing the case with general surgery, the patient underwent resection surgery without complications and uneventful hospital stay, and without signs of recurrence of herniation at the follow-up appointments.
Conclusion
No unified agreement exists on the best treatment of these complex high-flow lesions, and it is difficult to establish a comprehensive strategy given the pathology’s clinical variability, complex stratification, and the risk of relapse. A case-by-case approach is needed in managing these types of lesions.















