Introduction
Cerebrovascular disease is the most frequent cause of death in Portugal, accounting for 3322 deaths in 2016, 2096 of which correspond to ischemic strokes,1 and it also entails considerable morbidity and economic burden. For these reasons, the Portuguese national health system has deemed the prevention of cerebrovascular events a priority. Since 10-15% of patients have a previously undiagnosed and hemodynamically significant (>50%) internal carotid artery (ICA) stenosis,2 secondary prevention of thromboembolic stroke with carotid endarterectomy (CEA) of asymptomatic carotid stenosis has been considered a sensible option in selected cases.3
CEA is classified by the European Society of Cardiology (ESC) as an intermediate surgical risk procedure, meaning that the incidence of cardiac ischemic events and cardiovascular (CV) death in the 30-day postoperative is estimated at 1-5%.4 Other specific complications, like procedural (30-day) stroke or all-cause mortality are severe but very infrequent events, and guidelines have proposed a threshold of 3% as an acceptable perioperative risk for CEA,5 which should be audited independently.3 Recent Portuguese studies report death rates of 0.8-2% and stroke rates of 1-4%.1,6,7Other complications (tensional lability, cranial nerve injury, or cervical hematoma) with a higher incidence are usually mild and transitory, and only a restricted group requires treatment.
The most recent guidelines from the European Society for Vascular Surgery (ESVS) recommend 3-6 hours of close neurological vigilance and invasive blood pressure (BP) monitoring, followed by hourly non-invasive BP measurements and neurological monitoring for the first 24 hours after surgery.3 The lack of strong evidence for the suggested timelines and the little research dedicated to the early outcomes of CEA have given rise to multiple care modalities. However, considering the low rate of serious postoperative complications, only a restricted number of patients are likely to benefit from prolonged (more than 6 hours) stay in high-dependency units (HDU).3 Therefore, prolonged HDU stay presents multiple detrimental consequences for the patient and negatively impacts hospital resource management. Early transfer to the ward could be an alternative that would reduce the length of hospital stay, nosocomial infections, iatrogenic consequences associated with invasive monitoring, and overall patient costs. Considering all these factors, differentiating these two groups of patients (those who benefit from prolonged HDU stay and those who can be safely transferred to the ward) would allow more personalized care for each individual and increase the cost-effectiveness of the intervention.
A retrospective study was initially designed to identify patient characteristics and/or perioperative events that predispose to a higher benefit from prolonged HDU stay versus an early transfer to the ward.8 Increased intra-operative clamping time, increased preoperative systolic blood pressure (SBP), elevated maximum intra-operative mean blood pressure (MBP) and eversion technique were identified as predictive variables of the need for prolonged HDU stay.
The aim of this study was to replicate the previously described protocol in an independent center to externally validate the predictive variables of postoperative need for prolonged HDU stay.
Methods
Study design and patient selection
This is an observational retrospective study of consecutive patients, designed and executed according to Strobe guidelines for observational studies,9 performed in a tertiary-referral center, the Local Health Unit of São João in Porto, Portugal. The authors approached the Health Ethics Committee for consent for this protocol, which was granted. The protocol is registered and available for consultation on the public website ClinicalTrials.gov under the identifier NCT04327492.
The hospital clinical database was searched for all asymptomatic CEAs performed from January 2016 to December 2017. The ICD-9-CM diagnostic code used to identify the cases was 433.10, corresponding to ’Occlusion and stenosis of precerebral arteries and carotid artery, without mention of cerebral infarction’. Exclusion criteria were synchronous to cardiac surgeries, interrupted surgeries, surgeries performed in symptomatic patients (miscoded), or operations performed using the direct closure technique.
Patient characteristics
Demographic and clinical data were collected by retrospective patient medical records analysis. Age and sex categories were reported for all patients. Smoking habits were ascertained and sorted through three categories: patients who had never smoked, ex-smokers (patients who quit more than 2 months before the surgery), or active smokers (patients who were active smokers at the time of surgery or who had quit less than 2 months before the intervention). Other co-morbidities were accessed: diabetes mellitus (DM), obesity (defined as body mass index of more than 30kg/m2), arterial hypertension, coronary artery disease, congestive heart failure, arrhythmia (atrial fibrillation or flutter), peripheral artery disease, chronic pulmonary disease and previous (>6 months) stroke history concerning the anterior circulation and/or posterior circulation. The use of the following medications was documented: diuretics, beta-blockers, angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, statins, nitrates, and antiplatelet and anticoagulant drugs. Creatinine levels were attained from the preoperative laboratory workup. The ward’s non-invasive BP measurements were reviewed, and the one closest to the beginning of the surgery was selected and documented as basal BP.
Contralateral common carotid artery stenosis was ascertained and classified by duplex ultrasonography.10
Surgical procedure
CEA was either performed with patch closure or using the eversion technique.11,12 The surgery may be performed under general or regional anesthesia, with selective use of a shunt, if direct or indirect signs of cerebral hypoperfusion arise.
Intra and postoperative monitoring
The anesthesia monitoring software Anesthesia Manager® (PICIS Clinical Solutions Inc., Wakefield, MA, USA) gathered additional detailed information regarding the intraoperative patient status and the postoperative stay in the HDU. These included administration of intraoperative drugs such as volatile anesthetics, propofol, aminergic drugs (ephedrine, phenylephrine, atropine), beta-blockers and analgesic medication; cerebral oximetry value measurements using near-infrared spectroscopy (NIRS) (if multiple measurements were available the ones chosen for analysis were the highest value attained before clamping and lowest value after clamping); duration of the surgery; and clamping duration.
In the intraoperative period, the patient’s BP (arterial catheter), arterial blood saturation (oximetry), and cardiac electrical activity (electrocardiographic leads II and V5) were continuously monitored. A sample of measurements collected every 15 minutes was used for further analysis of the continuous monitoring. The following values were documented: maximum and minimum systolic BP (SBP) before and during induction of anesthesia; maximal mean BP (MBP), minimum SBP values, presence of bronchospasm, bradycardia episodes (heart rate < 60 bpm), and electrocardiogram abnormalities (ST-segment elevation or depression of 0.2 mv or more) during the entire intraoperative period. Major intraoperative cardiac and neurologic events were documented if present.
Complications
The postoperative complications considered were acute myocardial infarction (AMI), cranial nerve injury (CNI) (which was further differentiated into permanent or transient subgroups), cervical hematoma (with or without need for surgical drainage), bronchospasm, need for postoperative invasive mechanical ventilation, and neurologic events up to 30 days after surgery (major or minor ischemic stroke and symptomatic intracranial hemorrhage). To classify ischemic neurologic events, we used the criteria described by Rosenfield.13 Major stroke was defined as an increase in NIH Stroke Scale (NIHSS) score of >4 points or Modified Rankin Scale (MRS) score of >2 points from pre-stroke score or a stroke leading to MRS score of ≥5 points, persisting for at least 30 days or until next follow up visit. A minor stroke was a new stroke lasting longer than 24 hours that did not meet the criteria for a major stroke. Hemodynamic stability was accessed by the need for postoperative aminergic drug administration or antihypertensive/antiarrhythmic intravenous (IV) therapy. The same method described above was used to determine the maximum and minimum SBP values during the HDU permanence. Hospital stay time (through the admission and release dates) was also calculated.
Composite outcome (CO)
A surrogate marker, the CO, previously described to identify the patients who could benefit from prolonged HDU stay in the postoperative period was used.8 It was defined using the following variables: presence of intra and postoperative cardiac events, intra and postoperative neurologic deterioration, need for postoperative aminergic or ventilatory support or need for prolonged (more than 6 hours) antihypertensive IV therapy.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Science (SPSS Inc., Chicago IL, USA), version 29.0 for Windows®. Descriptive statistics are presented as absolute frequencies (n) and relative (%) for categorical variables. Continuous variables are expressed as mean and standard deviation (SD) when normally distributed and as median and interquartile range (IQR) when skewed. Differences between both groups were investigated using the Chi-square/Fisher’s exact test if the studied variables were categorical. For continuous variables, the t-test was used when normally distributed and Mann-Whitney U test for two independent variables when normality could not be assumed. Multivariable analysis resorting to logistic regression by the dimension reduction method was performed to address predictors of the CO. Variables that were statistically significant in the univariable analysis were included in the model to calculate odds ratios (OR) and 95% confidence intervals (CI).
To ascertain the validity of the previously described factors8 in an independent population, we used receiver operator characteristic (ROC) curve analysis of each of the statistically significant variables.
The tests were considered statistically relevant for a significance level (p-value) of less than 0.05 and the CI was 95%.
Results
Patient characteristics
Sixty-five surgeries performed in sixty-one patients were initially selected as potentially eligible for the study. A total of forty-eight patients and fifty-one CEAs met the inclusion criteria.
The sample was mainly composed of male patients (86%), and the mean age was 69±8years old, Table 1. Arterial hypertension was the most frequent comorbidity (94%) followed by DM (51%) and peripheral artery disease (51%). Smoking habits (either past or present) were documented in 61%. Twelve patients (24%) had a previous history of stroke, five ipsilateral to the endarterectomized carotid, five contralateral, and two involving the posterior circulation. Contralateral stenosis was present in 55% of patients, with an additional 15% with a history of prior carotid revascularization.
Table 1 Baseline sample characteristics of patients submitted to carotid endarterectomy due to asymptomatic stenosis
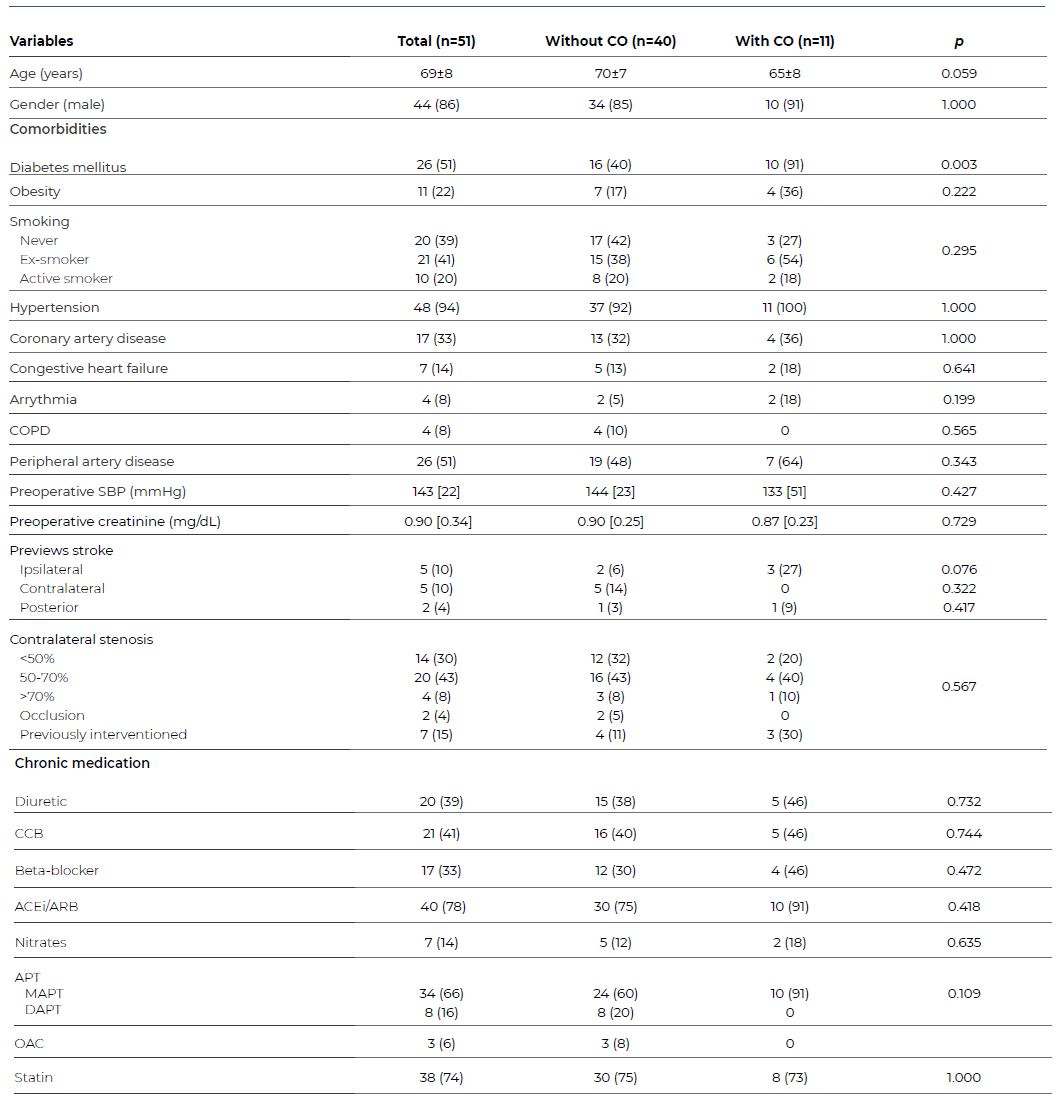
*Categorical data is presented as n (%); continuous data is presented as mean±standard deviation or as median [interquartile range] as appropriate. CO - composite outcome; COPD - chronic obstructive pulmonary disease; CCB - calcium channel blockers; ACEi - angiotensin-converting enzyme inhibitors; ARB - angiotensin II receptor blockers; APT - antiplatelet therapy; MAPT - mono APT; DAPT - dual APT; OAC - oral anticoagulant.
The optimal medical therapy for secondary prevention in asymptomatic patients at the time of the first observation in the hospital was monotherapy with aspirin (or clopidogrel as a second line antiplatelet agent) in 66%, statin therapy in 74% and 86% of the patients with hypertension were treated with one or more antihypertensive drugs. All patients were prescribed and adjusted to the best medical therapy during follow-up.3
Surgical and anesthetic procedures
Surgical and anesthetic details and other parameters are described on Table 2.
Table 2 Intra and postoperative technical and monitoring variables of patients treated with carotid endarterectomy for asymptomatic stenosis

Categorical data is presented as n (%); continuous data is presented as mean±standard deviation or as median [interquartile range] as appropriate. *Nitrous oxide and desflurane; **Ephedrine and phenylephrine; ***Labetalol and esmolol; CO - composite outcome; SBP - systolic blood pressure; MBP - mean blood pressure; ECG - electrocardiogram; HDU - high-dependency unit
Regional anesthesia was the preferred anesthetic technique (88%), and patch-closure repair (82%) was the preferred surgical technique. General anesthetic medication (propofol and volatile agents) was used in a small proportion of the sample (22%), and analgesia was achieved using acetaminophen and fentanyl (other opioids like morphine or tramadol had a marginal usage). Twenty-nine percent of patients needed intra-operative aminergic support, and 29% required antihypertensive medication (either intravenous labetalol or esmolol). The mean surgical time was 127 min, and the carotid artery was clamped for approximately 44 min. The cerebral oximetry detected a mean decrease of 10% after clamping the endarterectomized vessel and 1% after clamping the contralateral carotid. Two patients had an ipsilateral variation above 20%.
Hypertension was frequent during the procedure, especially during the pre-anesthetic period (median minimum SBP of 155mmHg). The overall intraoperative blood pressure was also elevated, with a median maximum MBP of 125 mmHg. Episodic bradycardia was perceived in 64% of patients, and 25% presented with ECG abnormalities.
In the HDU, the patients had a high variability of BP with a median maximum SBP of 164 mmHg and a minimum SBP of 89 mmHg. No episodes of bronchospasm were described during the intervention, HDU or ward stay. The median length of hospital stay after the procedure was three days with an interquartile range of two days.
Complications
Intra and postoperative complications are described in Table 3. The overall rate was 47%, and the most frequent event was CNI (n=9), occurring in 18% of the patients. However, only one case (2%) lasted longer than 30 days and had functional repercussions. Tensional lability also represented a large proportion of events, especially hypertension requiring IV medication for more than four hours, which affected six patients. This is congruent with the statistical difference of maximum SBP in the HDU (160 [41] vs. 195 [-], p=0.016) between the CO and the non-CO groups, given that it is a part of the compositive outcome.
Table 3 Baseline sample characteristics of patients submitted to carotid endarterectomy due to asymptomatic stenosis
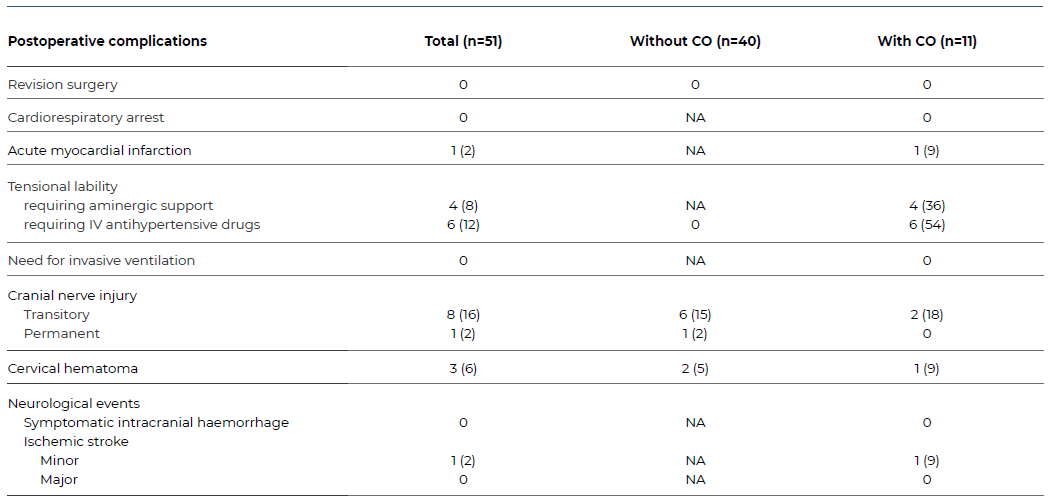
Categorical data is presented as n (%). CO - composite outcome; NA - non-applicable; IV - intravenous.
Cervical hematoma developed in 3 patients, and all cases resolved without further intervention. No patients needed invasive ventilation during the postoperative period.
In this study, the 30-day combined cardiovascular death or AMI rate was 2%, and the overall stroke rate was 2%. There were no cardiorespiratory arrests during the surgical procedures, and no patient presented with symptomatic intracranial bleeding on the postoperative period and up to 30 days.
Composite outcome
Based on these results, the authors identified 11 patients who presented with the CO. Tensional lability with the need for pharmacological intervention was the primary cause for CO inclusion (91% of cases). The most severe complications (intra or postoperative neurological deterioration or cardiovascular event) contributed with two of the 11 cases. One patient presented with more than one component of the CO needing both aminergic and antihypertensive support.
Length of hospital stay did not differ significantly between groups. Patients without the CO had a median stay of 3 days with an IQR of 2 day and patients presenting with the CO had a median stay of 4 days with an IQR of 4 days.
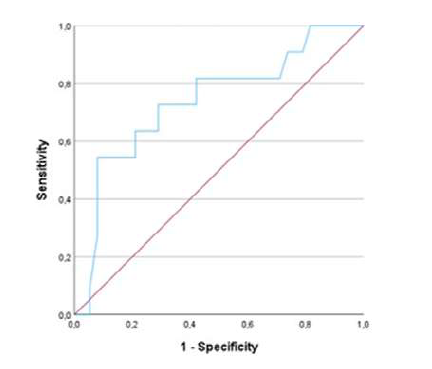
Concerning the previously described predictive factors of CO,8 only one reached statistical significance in our population (maximum intraoperative MBP: 124 [24] vs. 139 [31], p=0.017). Plotting of the ROC curve and curve coordinates are shown in Figure 1, demonstrating an AUC of 0.739 (0.564-0.914, p=0.017).
This study identified two additional predictors: DM and intraoperative analgesia with acetaminophen and maximum intraoperative MBP.
When added to the multivariate model, the adjusted OR (95% CI) was 15.0 (1.7-128.9, p=0.014) for DM and an OR of 0.07 (0.01-0.42, p=0.004), for the use of acetaminophen.
Discussion
The presented work is a retrospective cohort study using a consecutive sample that focused on asymptomatic patients submitted to CEA in a tertiary-referral hospital in the North of Portugal aiming to externally validate a protocol designed and implemented in another Portuguese hospital center, which included a CO to serve as a surrogate marker for the need for prolonged HDU stay. From the described CO predictive factors, only maximum intraoperative MBP showed a statistical association in both analyses. Other factors (eversion technique, carotid clamping time, maximum intraoperative SBP and volatile anesthetic utilization) did not reach significance. Some may be explained by the different practices between hospitals (especially concerning anesthetic technique, predominantly regional in this sample, and so there is a marginal use of gas anesthetics), and the small sample size might justify the absence of effect for the others. Even though the two hospital centers are located in the same region of Portugal, the population characteristics may differ, resulting in the observed discrepancy in predictive factors.
Asymptomatic CEA is a prophylactic intervention that aims to prevent the occurrence of stroke in high-risk individuals. Nevertheless, CEA is not free of peri-operative risks. It is then imperative to ensure that the benefit outweighs the inherent risks through high-quality care during the perioperative period. At 30 days, the sample described in the study had a 2% stroke/death rate and a cardiovascular death or AMI of 2%, assuring the quality standards recommended. Temporary CNI reached 18% prevalence, but the rate of permanent CNI (2%) found in the sample was comparable to the literature (1-6%).6,13-15
The rates of the remaining minor complications (cervical hematoma and hemodynamic lability requiring IV medication) were similar to those described in the literature.6,14-18 Furthermore, the overall average length of hospital stay was comparable to and even lower than those described in our national setting.6,19
The CO included 11 patients who developed an adverse event that would be better managed in an HDU and so these were the ones who benefited from the permanence in this typology of units. Given that there are one-day hospital stay protocols implemented in multiple centers14,20-24 with the same or lower complication rates, the question arises of whether this increase in HDU and hospital stay benefits the patients. The CO was present in 22% of the sample, and the most frequent event was the need for >4 hours of IV medication for hypertension, followed by the need for aminergic support, stroke, and AMI.
In the first center where the protocol was established, a 55% incidence of CO was attained. The surplus was primarily due to the higher proportion of patients needing prolonged anti-hypertensive IV therapy (45% versus 12% in our population). These findings should prompt a reflection on whether practice and protocol (lower BP targets or thresholds for hypertension treatment) could explain this disparity.
Reviewing the preoperative characteristics analyzed in this paper, DM was the only one that showed a significant association with the CO and respective adverse events. DM has long been proven to be a major cardiovascular risk factor, increasing the odds of disease by 2-4-fold(25, 26). Hyperglycemia and its underlying cellular pathway abnormalities cause chronic inflammation and endothelial dysfunction, leading to impaired vasodilation and thrombotic and proliferative effects that significantly accelerate the formation and progression of atherosclerotic lesions.25,27,28 This association makes macrovascular complications, such as ischemic heart disease, cerebrovascular accidents, and peripheral vascular disease, the most important cause of morbidity and mortality in patients with type 2 diabetes.25,26,29,30 For the reasons explained above, diabetics are a particularly suitable group for prophylactic intervention.
The impact of DM on early CEA outcomes has been a long-debated topic and has generated controversy that is yet to be settled. Multiple studies, both observational and clinical trials, have demonstrated increased odds of several intra and postoperative adverse outcomes, from CNI and surgical site infection to cardiac and neurological events. It has also been associated with prolonged length of hospital stay and early and long-term increased mortality.14,26,31-35
On the other hand, according to a 2018 observational study with 314 CEAs, diabetic patients did not demonstrate higher stroke risk at 36 months of follow-up.36 Earlier published articles showed the same absence of poorer prognosis for diabetic patients but highlighted that the lack of significant difference may be due to the low frequency of major events.37,38 Jeong et al., using the same methodology and including 675 patients, reached the same conclusion when it comes to early outcomes but had a higher risk of stroke at four-year follow-up Mixed symptomatic and asymptomatic samples and single center population of the studies mentioned above pose obstacles to comparability with our case.39
The work presented in this paper establishes DM as a predictor of the CO. Although this finding is insufficient to answer the pending question, it is another piece of evidence favoring the association between postoperative complications and DM. The significant association with DM in such a small sample may be partially explained by the inclusion of tension lability needing IV therapy in our CO.
The maximum MBP during the procedure was also associated with the CO. Preoperative hypertension has long been known to pose the risk for postoperative complications.40,41 Accordingly, guidelines propose preoperative BP control in a chronic or acute setting and a preoperative SBP > 180mmHg should be considered for immediate and intensive anti-hypertension treatment when surgery is urgent or delayed until BP is adequately managed.
The mechanisms for perioperative hypertension have been extensively described. The carotid bulb denervation during surgery and stress hormone production (renin and norepinephrine)42-44 are some of the hypotheses suggested and will show their effect still in the operating room. This blood pressure dysregulation usually normalizes in 24 hours, but up to 50% will need IV anti-hypertensive medication in the postoperative period. Intraoperative hypertension is then, not surprisingly, a marker for postoperative hypertension and our CO. This enforces the need for tight BP control during surgery to ensure lower risk for the need for HDU permanence.
Where acetaminophen is concerned, after reviewing the literature, no previously documented association was found between the drug and a protective effect against postoperative complications. This represents either a new finding needing further investigation or may be due to confounding by an undetermined factor.
The hypotensive effect of IV acetaminophen is extensively described in retrospective45 and prospective46-48 observational studies and randomized clinical trials.49,50 Although most of these were set in intensive care units with critical patients, the same effect has been observed in healthy volunteers.50 The mechanism is not entirely understood, but Krajcova et al. suggested it was related to an acetaminophen-induced decrease in cardiac output and systemic vascular resistance.48 This blood pressure drop may affect as much as 50% of patients and showed clinical significance with need for intervention in 11-16%. All things considered, it is possible that anesthesia teams avoid using this drug in patients that they predict, through clinical experience and patient status and comorbidities, to have a higher risk of developing hemodynamic instability, giving way to a false association. From another perspective, one might hypothesize that the hypotensive effect may be beneficial in patients with a hypertensive profile, albeit the fact that the blood pressure decrease usually happens in the first 15 to 30 minutes.46,47,50 Finally, adjusting BP measurements during anesthetic induction and operation did not change the association of acetaminophen and the CO, making these explanations less likely.
This study is not without limitations. The sample is small and, since major postoperative events after CEA are rare, it might be underpowered to find and extensively define more predictors of the CO. The single center population contributed to the low number of patients and poses a generalization impediment. Cautious extrapolation of results could be acceptable for similar health care facilities and populations. The retrospective nature of the research also adds several possible restrictions. Besides the suspected confounding factor previously exposed, multiple information biases can occur in this setting, namely misclassification, observer, and reporting bias. Bearing in mind the complex character of clinical documentation (various, sometimes specialty-specific, data storing software, input from different types of health professionals, team fluctuation) and seeing that the data source was restricted to informatic clinical files, it is impossible to ascertain the full weight of these conditioning elements.
In conclusion, asymptomatic CEA is a safe procedure with rare early postoperative complications. The implemented HDU prolonged stay does not seem to bring benefit to most patients, except for diabetics who are prone to complications better managed in a more differentiated unit than the ward. Although the results are insufficient to elaborate a protocol to guide patient care and orientation, these findings reinforce the need for further investigation on this topic and confirm the necessity for standard care revision.