Introduction
Fenestrated and branched endovascular aortic repair is widely used to manage complex aortic aneurysms, reducing morbidity and mortality compared to open surgical repair.1-5 This technique incorporates mesenteric and renal arteries using reinforced fenestrations or directional branches, ensuring complete aneurysm exclusion and obtaining an adequate proximal seal. Dr Benjamin Starnes introduced the term "physician-modified endograft" (PMEG) to describe the adaptation of commercial aortic stent grafts via back-table modifications to include custom fenestrations or branches.6 This method is particularly suited for rapidly repairing large, symptomatic, or ruptured aneurysms. Although the use of PMEG is increasing, research still needs to be improved, given the frequency of its off-label use. A 2023 study by Chait J. et al. evaluated 89 patients with complex aortic artery aneurysms; 15% were symptomatic or had ruptured aneurysms. The study reported a 99% technical success rate, a 2% early mortality rate, and a 19% rate of major adverse events. Additionally, it showed a 95% ± 3% rate of freedom from aortic-related mortality and a 45% ± 7% five-year survival rate.6 In 2024, Tsilimparis et al. presented results from a multicentre study involving 1274 patients across 19 centers, 45.7% of whom had complex abdominal aortic aneurysms, including elective, symptomatic, and ruptured cases. The overall technical success rate was 94%, with a 30-day mortality rate of 5.8%. Major adverse events occurred in 25.2% of cases. Freedom from reintervention was 73.8%, 61.8%, and 51.4% at 1, 3, and 5 years, respectively, while primary target vessel patency was 96.9%, 93.6%, and 90.3%. Overall survival and freedom from aortic-related mortality were 82.4%/92.9%, 69.9%/91.6%, and 55.0%/89.1% at 1, 3, and 5 years, respectively.7 Tachida et al. focused on reinterventions following PMEG procedures, finding that most were percutaneous, minor, and low magnitude, with no significant impact on long-term survival. However, early reinterventions were associated with an increased mortality risk.8
The parallel graft technique (chEVAR) is an alternative for urgent endovascular treatment of complex aortic aneurysms. A long-term analysis from the PERICLES registry reported primary patency rates for chimney grafts of 94%, 93%, 92%, and 90% at 2.5, 3, 4, and 5 years of follow-up, respectively.9 However, type 1A gutter-related endoleak and target vessel occlusion remain significant concerns, with the risk increasing proportionally to the number of chimney grafts used. Scali et al. found that the hazard ratio for chimney graft occlusion increases by 1.8 for each additional graft.10 Consequently, guidelines recommend using chEVAR primarily in emergency settings or as a bailout, ideally limiting the procedure to one or two chimneys.11
Case report
Patient Information
We report the case of a 69-year-old Caucasian male with a history of smoking, hypertension, and dyslipidemia. He was previously diagnosed with a 46 mm infra-renal aortic artery aneurysm (AAA) via ultrasound six months prior. He had no significant familial or psychosocial history.
Clinical Findings
The patient was transferred to our hospital from another healthcare facility due to severe, persistent hypogastric pain over four days. At admission, he was hemodynamically stable with mild hypertension and presented with abdominal tenderness but no other gastrointestinal symptoms. Laboratory evaluations, including blood tests and gas analysis, were normal.
Diagnostic Assessment
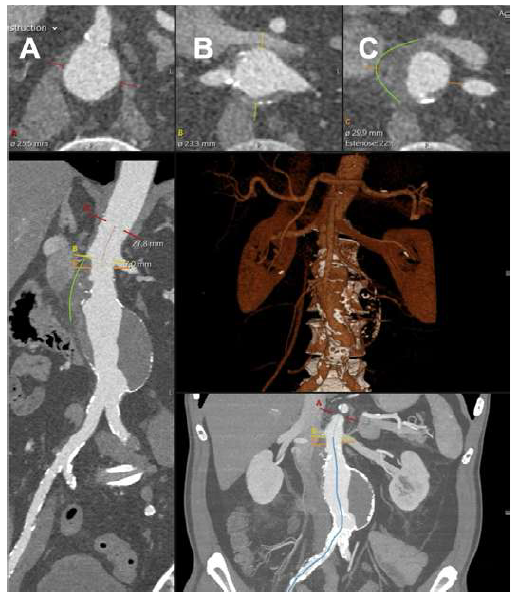
Given the patient’s sudden abdominal complaints and a previously identified AAA, a computed tomography angiography (CTA) was performed. The CTA revealed a 60 mm juxta-renal abdominal aortic aneurysm with signs of instability, characterized by parietal contour discontinuity at the proximal neck without active contrast extravasation (Figure 1).
Therapeutic Intervention
Pain management was effectively achieved with opioid analgesia and hypertension control. The patient was then transferred to the Intensive Care Unit for continuous monitoring and was scheduled for an urgent, deferred repair.
Following a thorough preoperative clinical and radiological assessment, an endovascular aortic repair (EVAR) was considered the most suitable treatment for this patient.
Due to the lack of off-the-shelf branched aortic devices at our center and the complications associated with three grafts in a chimney EVAR (chEVAR) that this patient would require, we opted for a PMEG. This custom-made device included fenestrations for renal arteries and a scallop for the superior mesenteric artery.
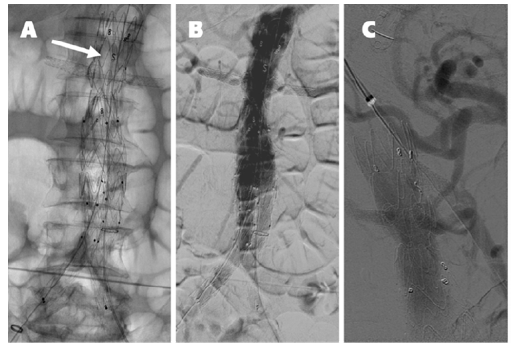
A 30 mm thoracic aortic endograft platform (Valiant Captivia, Medtronic®) was deployed with renal fenestrations positioned at 3 and 9 o’clock (Figure 1), crafted with thermocautery. The modifications were reinforced with pushable coils and secured with a continuous polypropylene suture (Video 1). To facilitate precise orientation upon implantation, one of the endograft’s proximal radiopaque markers was reshaped into an ‘S’ configuration and re-implanted on the graft’s anterior aspect (Figure 2, arrow on panel A). No diameter-reducing ties were employed. The graft was re-sheathed using a strangling silk technique (Video 2). PMEG preparation was completed within 90 minutes.
The top panels show the superior mesenteric artery (panel A), renal arteries (panel B), and aneurysm origins (panel C). The green lines outline the signs of instability in the neck.
We performed the procedure in the standard operating room with a portable imaging unit (Vision RFD Hybrid Edition, Ziehm Imaging®). After appropriate preparation and draping, the right common femoral artery was surgically exposed, and percutaneous access to the left femoral artery was secured under ultrasound guidance.
Although not seen, the superior mesenteric artery is patent. The arrow on panel A indicates the ‘S’ shape radiopaque marker implanted on the anterior aspect of the graft for position reference. Panel C shows the completion angiography after the balloon-expandable stent graft deployed via the upper-arm approach in the superior mesenteric artery.
A contrast aortogram was performed using 20 mL of dilute contrast injected at a rate of 25 mL/s, with the PMEG in place to delineate the visceral vessels. The proper orientation of the graft was verified by rotating the device to ensure the correct positioning of the ‘S’ shaped marker. The PMEG was unintentionally deployed slightly above the intended landing zone, which complicated but did not preclude renal artery cannulation. The renal arteries were accessed using a tri-axial system consisting of a 6.5 Fr steerable sheath (TourGuide APTUS, Medtronic®), a 5 Fr Berenstein diagnostic catheter, and a hydrophilic guidewire. After cannulation, an 8 Fr sheath was advanced into each renal artery, facilitating the secure delivery of 6 mm stent grafts (Radiant, Medtronic®). Both renal stent grafts were deployed simultaneously and then proximally flared using a 10 mm balloon (Admiral Xtreme, Medtronic®).
Standard EVAR techniques facilitated the distal bi-iliac repair (36 mm main body, Endurant IIs, Medtronic®). A completion aortogram was performed at the end of the procedure (Figure 2, panel B), which was completed in 145 minutes, involving the administration of 120 mL of contrast and a cumulative fluoroscopy time of 78 minutes. Control CTA on the following day identified partial occlusion of the superior mesenteric artery ostium caused by the graft scallop. This issue was resolved via an upper-arm approach by deploying an 8 mm balloon-expandable endograft (Viabahn VBX, Gore Medical®) (Figure 2, panel C).
Follow-up and Outcomes
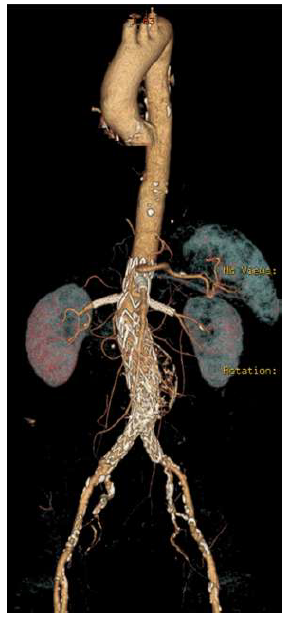
The patient was discharged home on the ninth postoperative day. A six-month follow-up CTA confirmed the AAA's exclusion, with maintained patency of all visceral vessels and no evidence of endoleak (Figure 3). No further clinical events were related to the AAA or the PMEG.
Complete exclusion of the juxta-renal aneurysm is noted, with no evidence of endoleak, and patency of all target visceral vessels is maintained.
Conclusion
Fenestrated PMEG presents a safe and effective option for the urgent management of juxta-renal abdominal aortic aneurysms that require renal artery preservation. Modifying endovascular aortic repair grafts is a highly technical process that demands meticulous planning and extensive experience in fenestrated endovascular aortic repair.