Introduction
Abdominal aortic aneurysms (AAA) are a disease most found in male smokers over the age of 65. Risk factors typically associated with worse cardiovascular outcomes, such as male sex, hypertension, and a history of smoking, account for most cases and for a higher rate of disease progression. Its prevalence can range from 1% to 5% in this population. Although there has been a decline in admissions, the incidence of death from ruptured AAA can still reach 10 per 100 000.1,2 Paradoxically, various meta-analyses identify diabetes mellitus as a protective factor against the development of AAA.
This hypothesis has been tested at a molecular level, providing evidence of a different inflammatory profile.3In animal models, diabetes correlates with lower levels of matrix metalloproteinases, which are usually responsible for the medial degeneration of the aortic wall.4 In the clinical setting, diabetic patients seem to have a lower incidence of the disease and a slower aneurysm growth rate.
Furthermore, regarding the risk of rupture, a recent study found no protective effect of diabetes, although it does not increase the mortality rate either.5
Despite the findings in current literature, little is known about whether this protective effect is related to the disease or the use of antidiabetic drugs, such as metformin.3,6,7 Moreover, most studies primarily focus on AAA progression before intervention, with little to no evidence on postoperative outcomes following aortic repair in patients with type 2 diabetes, nor the effect of antidiabetics for these patients.
A nationwide survey in Scandinavia followed 2217 patients who underwent aortic repair, of which 343 (16%) had a previous diagnosis of type 2 diabetes. In this sample, diabetic patients were more likely to have a history of hypertension, hyperlipidemia, or other cardiovascular risk factors, although there was no statistically significant difference among the groups.8 Regarding ruptured cases, the authors report a lower proportion in diabetic patients (62% vs. 67%; p<0.05) and a lower postoperative mortality in ruptured aneurysms in diabetic patients (Relative Risk 0.65; 95% Confidence Interval [CI], 0.48-0.87; p=0.003).8
However, this study has several limitations, including the lack of data specifically regarding aortic events after surgery and the influence of antidiabetic drugs on morbidity and mortality.
As there is a surge in novel antidiabetic drugs with a clear cardiovascular benefit, namely SGLT-2 inhibitors, in vitro and animal models show promising preliminary results on a molecular level,9 it is of great importance to further characterize the impact of type 2 diabetes and particularly antidiabetic drugs in overall and aortic-related outcomes after endovascular AAA repair.
We hypothesize that diabetes may confer a protective effect after EVAR, with a lower rate of aortic-related complications such as rupture, endoleak or need for reintervention. Moreover, we hypothesize the protective effect may be more significant in patients taking oral antidiabetics (versus insulin).
Methods
This study followed the reporting guidelines from the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Statement for cohort studies.10
Study design
We performed a retrospective cohort study from a pool of patients who underwent elective surgical repair for abdominal aortic aneurysm at a tertiary hospital center. Patients to be included must be over 18 and subjected to EVAR for an intact infrarenal AAA. We excluded patients with type IV thoracoabdominal aortic aneurysms, pararenal or juxtarenal aneurysms or interventions involving renal and visceral branches/fenestrations/chimneys.
Symptomatic and ruptured AAA were also excluded from this analysis. In this study, eligible patients were categorized according to the presence of type 2 diabetes, either mentioned or diagnosed when in the presence of a fasting plasma glucose greater than 126 mg/dl, occasional plasma glucose greater than 200 mg/dL or HbA1c levels greater than or equal to 6,5%.
Study protocol
Eligible data regarding endovascular aortic surgery was collected from physical and electronic surgical records between January 2013 and April 2022. The start of follow-up was defined by the date of the index surgery. All patients were reevaluated at one week, one month, six months, and yearly after discharge. Control computed tomography (CT) scans were performed one month and one year after the index surgery following recent guidelines11, to assess endoleaks and other complications11 Annual CT scans were performed if a type 2 endoleak was identified. In the remaining cases, follow-up was conducted through serial ultrasonography.
Baseline assessment:
For each patient, data was collected on demography and comorbidities: the proportion of type 2 diabetes (exposure of interest), the proportion of cardiovascular and concomitant AAA risk factors, namely arterial hypertension, heart failure, acute coronary syndrome (defined as previous acute myocardial infarction events or evidence of coronary heart disease in previous angiographies), cerebrovascular disease (defined as previous stroke events or transient ischemic attacks, as well as previous carotid interventions), chronic renal disease (defined as an estimated glomerular filtration rate below 60 ml/min/1.76 m2 according to the modified MDRD equation), dyslipidemia, malignancy, previous abdominal or aortic surgeries and smoking habits.
Baseline medication, including oral antidiabetics (classified by their mechanism of action), antihypertensive drugs (specifically betablockers, ACEIs and ARAs), statins, antiplatelet drugs, anticoagulant drugs. AAA were morphologically characterized through thorough measurement of preoperative CT scans.
Study endpoints
The primary endpoint was defined by the incidence of aortic-related events, i.e., aortic reinterventions or ruptures, and related mortality. Secondary endpoints comprised efficacy measures such as sac diameter variation (in cm/year), calculated by the difference between the preoperative aneurysm diameter and postoperative aneurysm diameter at a given timepoint; endoleak rates; and overall and 30-day mortality.
Statistical analysis:
The study cohort was divided into two groups, as previously mentioned. We performed a descriptive analysis of our data. Continuous variables were presented as mean (standard deviation) if normally distributed and median (interquartile range) if not. Dichotomous and categorical variables were expressed in numbers (percentage). Two-sample t-test or Mann-Whitney test was used when comparing continuous variables and Chi-Square/Fisher’s exact test to compare dichotomous variables. Median follow-up time (in months) was assessed for both groups. Univariable and multivariable analysis was performed through Cox proportional regression models in a step-forward fashion to adjust for competing and potentially confounding factors (sex, age, dyslipidemia, arterial hypertension, chronic kidney disease). We considered statistically significant variables from the univariable analysis as well as variables relevant according to subject matter knowledge.
Crude and adjusted hazard ratios (HR) for the exposure of interest and significant covariables were reported. All analyses were considered statistically significant if a two-tailed p-value < 0.05 was observed. Statistical analysis was carried out using STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Results
Study population
The final analysis included 381 patients from surgical activity between 2013 and 2022 at a tertiary hospital center. Most patients were excluded for alternative diagnoses or aortic aneurysms from other topographies.
Table 1 details the baseline characteristics of the study cohort. The study population mainly comprises male patients, and the median age at intervention was around 75 years.
Regarding the overall cohort, diabetic patients represented 17.9% (14.6%-21.2%) of the total sample. Within this group, the majority of patients were taking metformin, while 6.5% used insulin. Overall, diabetic and non-diabetic patients had similar median aortic diameters. Comorbidities were evenly distributed between diabetics and non-diabetics, except for dyslipidemia, the use of statins, and renin-angiotensin-aldosterone blockade agents, all of which were more prevalent in diabetic patients, Table 1.
Table 1 Baseline characteristics of patients diabetic and non-diabetic EVAR patients, included in the study
| Diabetic (n=82) | Non-diabetic (n=299) | p value | |
|---|---|---|---|
| Male sex - N (%) | 74 (90.2) | 273 (91.3) | 0.77 |
| Age in years - Median (IQR) | 75 (70-81) | 76 (71-82) | 0.42 |
| Hypertension - N (%) | 78 (95.1) | 252 (85.1) | 0.02 |
| Smoking - N (%) | 49 (59.8) | 189 (63.9) | 0.50 |
| Dyslipidaemia - N (%) | 75 (91.5) | 220 (74.3) | 0.01 |
| Chronic kidney disease - N (%) | 24 (29.3) | 72 (24.1) | 0.34 |
| Chronic heart failure - N (%) | 20 (24.4) | 49 (16.4) | 0.02 |
| Coronary artery disease - N (%) | 27 (32.7) | 97 (32.4) | 0.76 |
| Chronic obstructive pulmonary disease - N (%) | 18 (21.9) | 57 (19.1) | 0.58 |
| Antiplatelet drug therapy - N (%) | 49 (60.3) | 191(63.9) | 0.57 |
| Anticoagulant therapy - N (%) | 20 (24.4) | 52 (17.2) | 0.14 |
| Statin therapy - N (%) | 67 (81.7) | 210 (70.2) | 0.03 |
| ARB/ACE-I therapy - N (%) | 65 (79.4) | 192 (64.3) | 0.01 |
| CCB therapy - N (%) | 26 (31.7) | 84 (28.1) | 0.52 |
| Beta-blocker therapy - N (%) | 33 (40.2) | 102 (34.1) | 0.27 |
Incidence of postoperative aortic events and surgical outcomes
Patients were followed up for a median of 23 (6-40) months. Regarding the primary outcome, diabetic patients presented a higher incidence rate of aortic events, although without reaching statistical significance, Figure 1. After adjusting for confounding factors, diabetes kept a non-significant association regarding the incidence of aortic events, adjusted HR (aHR) 1.21 (0.64-2.30), Table 2.
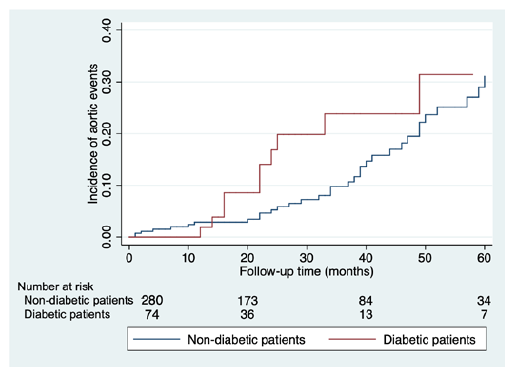
Figure 1 Kaplan-Meier survival analysis of aortic events during the study follow-up, comparing diabetic and non-diabetic EVAR patients.
Table 2 Cox proportional hazard regression model for the incidence of aortic events and effect size of diabetes
| Hazard ratio (95% CI) | |
|---|---|
| Crude HR | 1.55 (0.83-2.91) |
| Adjusted HR | 1.39 (0.71-2.72) |
| Hypertension | 1.34 (0.55-3.29) |
| Age | 1.01 (0.97-1.04) |
| Female sex | 1.40 (0.59-3.32) |
| Chronic kidney disease | 0.54 (0.27-1.06) |
| Smoking | 0.91 (0.51-1.66) |
| Dyslipidemia | 1.27 (0.67-2.41) |
HR - hazard ratio; CI - confidence interval
Most aortic events corresponded to reinterventions, with a median time of 39 (24-60) months from the index surgery. Most reinterventions were related to type 1 endoleaks (28.3%), requiring proximal extension in six cases and iliac extension in nine cases.
Nine patients (17%) underwent reintervention during follow-up due to type 2 endoleak with significant sac expansion. Most of these type 2 endoleaks were corrected through open surgery with lumbar artery ligation, while two patients underwent laparoscopic inferior mesenteric artery ligation. Two patients had reinterventions for type 3 endoleaks. Regarding limb complications, seven patients, of which five were diabetic, underwent reintervention due to limb occlusion or stenosis.
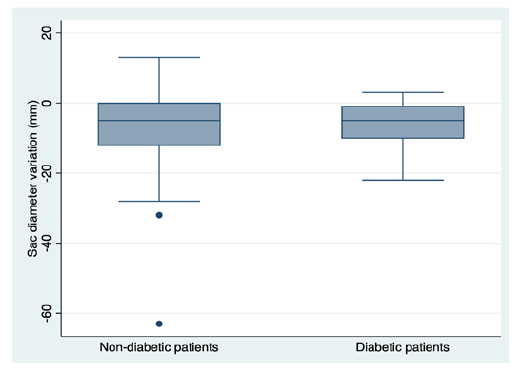
Regarding secondary outcomes, mortality rates were significantly higher in diabetic patients (aHR 1.86, p=0.02), Figure 2. We reported non-significantly higher rates of type I and II postoperative endoleak in diabetic patients (7% vs. 5%, p=0.55 and 29.6% vs. 22.0%; p=0.17, respectively). When assessing the effect of diabetes on sac dynamics, there was a non-significant trend for lower mean sac shrinkage in diabetic patients (-5.26mm ± 10.48mm vs. -6.85mm ± 5.80mm, p=0.41), Figure 3.
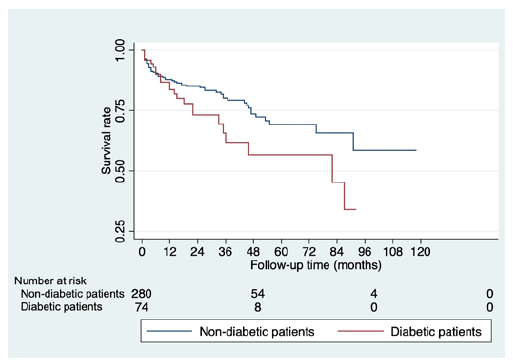
Figure 2 Kaplan-Meier survival analysis of overall mortality during the study follow-up, comparing diabetic and non-diabetic EVAR patients.
Discussion
Our main findings point to a negative, although non-significant effect of diabetes on postoperative outcomes after endovascular and open aortic repair. This conclusion was also identified in a recent nationwide study in France, which reported a non-statistical association between type 2 diabetes and aortic mortality and events.12 Diabetes appears to be a protective factor for AAA growth and rupture in a preoperative setting. Ning et al., in a prospective analysis using data from the Atherosclerosis Risk in Communities (ARIC) Study, reported significantly smaller aortic diameters.13 This result was particularly true for long-duration diabetes.13 In our study, diabetes did not seem to have a particular effect on AAA rupture, with similar rates to non-diabetic patients. This conclusion was also drawn in a matched case control study from Denmark. Kristensen et al. reported a non-significant effect size of diabetes on AAA rupture, even after adjusting for several confounders.5
On the other hand, an observational study from Taimour et al. reported an overall lower risk of rupture in diabetic patients.5 A different inflammatory profile in diabetic patients, with increased aortic wall stiffness, is one of the proposed mechanisms for this lower rate of complications.14,15Moreover, a lower preoperative aortic diameter in diabetic patients would likely contribute to an overall risk of sheer stress and aortic rupture.16
In this cohort, diabetes did not have a significant impact on the rate of postoperative aortic events. Nevertheless, our results pointed towards a non-significant trend for higher rate of reinterventions and aortic-related mortality in diabetic patients. This result was contrary to the conclusion of a prospective cohort study performed by Png et al., focused on patients submitted to EVAR. These authors reported a lower rate of aneurysm sac enlargement and a trend towards fewer reinterventions.17 This higher rate may be explained by a higher proportion of dyslipidemia and chronic heart failure, which increases the atherosclerotic burden in these patients.
Aneurysm sac shrinkage was identified as a marker of technical success for EVAR. However, according to a systematic meta-analysis performed by Lalys et al., type 2 diabetes appears to have a negative, although non-significant effect on sac shrinkage.18 In this study, there was a trend for a lower sac shrinkage in diabetic patients and a non-significantly higher rate of late type 1 and 2 endoleak. Recent data shows no significant association between the rate of these complications and diabetes. Indeed, a five-year follow-up on patients in the GREAT (Global Registry for Endovascular Aortic Treatment) registry shows no statistically significant difference in endoleak and reintervention rates, although there is a non-significant trend for lesser sac regression in diabetic patients.19 Type 2 endoleak results from the persistent flow from anatomical factors that maintain sac perfusion despite a success endovascular exclusion.20 Two systematic meta-analyses identify patent lumbar arteries or the inferior mesenteric artery as main contributors for this type of endoleak, reflecting increasing trends for pre-emptive embolization of these vessels during an EVAR procedure.21,22 There are no studies reflecting a hypothetical anatomical variation in diabetic patients. When type 1 and 3 endoleaks are concerned, few studies report the potential impact of diabetes on these complications. A group from Adelaide developed an EVAR risk assessment model based on prospective data collection from patients submitted to EVAR between 2009 and 2013. The group led by Cowled reported a significant protective effect of diabetes, with lower rates of early type 1 and 3 endoleaks, although no mechanism or possible explanation was provided to these findings.23
This study has the strength of thoroughly evaluating all types of postoperative events for a non-selected elective EVAR population. Furthermore, the inclusion of patients between 2013 and 2022 follows the temporal trends and evolution in current practice. Nevertheless, because of this retrospective nature, this study presents several limitations. Besides a recall bias, the low proportion of diabetic patients in the cohort may underline a higher proportion being followed up for smaller aneurysms, thus with no surgical indication. As there was a small sample of diabetic patients, no inferences could be performed regarding the effect of antidiabetic drugs on postoperative outcomes.
Conclusion
Although diabetes presents a protective effect regarding AAA progression and rupture, this condition seems to have a negative, although non-significant effect on postoperative outcomes, namely aortic reintervention and mortality. Diabetic patients presented higher rates of postoperative endoleak, contributing to a lower sac shrinkage rate. Further studies should aim to identify the role of antidiabetic drugs in the incidence of aortic reinterventions. There may be suitable agents to mitigate the rate of complications in these patients. Ultimately, the role of diabetes in aortic aneurysmal may be more complex than expected and share common features with other forms of peripheral artery disease.