Background
Carotid pseudoaneurysms are extremely rare, and their inherent instability and unpredictability require prompt evaluation and intervention to mitigate potential neurological complications or prevent life-threatening bleeding and airway obstruction. Etiology may include infection, tumor invasion, vasculitis, plaque rupture, iatrogenic injury, blunt trauma or radiation necrosis.1,2 We present a case report of spontaneous internal carotid artery (ICA) rupture.
Case report:
An 88 year-old autonomous but frail male with a history of non-Hodgkin lymphoma and adenocarcinoma of the rectum (T3 N0 M0) under radiotherapy, presented with sudden-onset left cervical pulsatile mass and dysphagia. The patient had no trauma or history of previous surgical intervention on the neck. In the analytical study at admission, the patient had leukocytosis (leukocytes 15.83x103 with 40.7% neutrophils) and a C-reactive protein level of 79 mg/L. The computed tomography angiography (CTA) revealed a left internal carotid artery rupture with a slight deviation of the trachea (Figure 1).
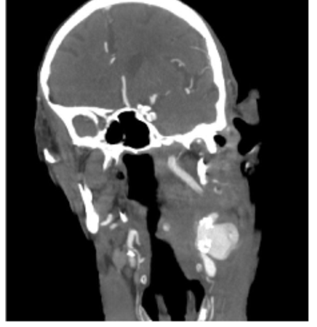
Figure 1 Computed tomography angiography at admission showing a carotid rupture within a calcified atherosclerotic plaque and hematoma compressing the cervical structures (coronal view)
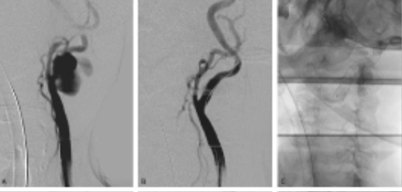
The patient was immediately taken to the operating room, where airway protection was achieved through orotracheal intubation, and the bleeding was controlled through pseudoaneurysm exclusion with a stent graft, Figure 2. Carotid artery access was achieved through a transcervical surgical approach with a suprasternal transverse incision, and an 8Fr sheath was placed in the common carotid artery (CCA). Angiography revealed the rupture in the proximal ICA, Figure 2A. The ICA was catheterized with a .035” hydrophilic guidewire, and a 7x50 mm self-expandable stent graft (Viabahn®) was deployed, with immediate resolution of the mass pulsatility, Figure 2B and 2C.
extravasating contrast from the ruptured site at the origin of the internal carotid artery; B) completion angiogram after exclusion with a self-expandable stentgraft; C) 7x50 mm Viabahn® deployed from the origin of the internal carotid artery.
Since the patient did not present with respiratory distress or stridor, and the videolaryngoscopy did not show a significant reduction in the laryngeal lumen, it was decided not to proceed with surgical drainage of the hematoma due to the additional morbidity associated with this procedure.
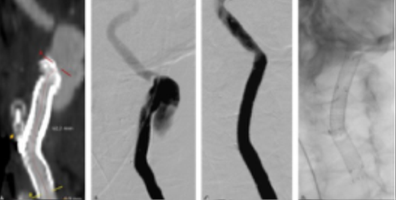
Control CTA at 24 hours revealed a "type 1 endoleak" with contrast leak filling from the proximal landing zone, Figure 3. A second procedure was performed through a transfemoral approach. An 8Fr 70cm sheath was placed at the origin of the CCA through a right percutaneous femoral access. The external carotid artery ostium was occluded with an 8mm vascular plug (Amplatzer™ Vascular Plug 4, 8x13.5), followed by stentgraft extension to the common carotid artery with an 8x50 Viabahn®, Figure 4.
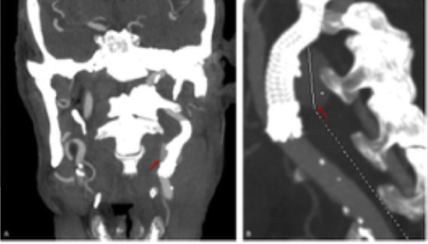
Figure 3 Control computed tomography angiography showing a contrast leak (red arrows) from the proximal landing zone. A) coronal view; B) sagittal view.
The patient had progressive resolution of symptoms with no need for surgical decompression. Empiric antibiotic therapy was initiated with amoxicillin and clavulanic acid at admission, which was escalated to piperacillin-tazobactam after 48h because of documented fever under the initial antibiotic scheme. Blood cultures collected at admission were positive for Pseudomonas aeruginosa with intermediate sensitivity for piperacillin-tazobactam and quinolones, turning negative in the set collected 24 hours after escalating antibiotics.
The patient underwent a 21-day course of intravenous antibiotics with piperacillin-tazobactam, transitioning to oral levofloxacin upon discharge, according to the antibiogram. The antibiotic therapy was suspended by the 9th week after a multidisciplinary discussion with the infectiology team, with sustained negative blood cultures and complete resolution of symptoms, Figure 4.
By the 5th month of follow-up, the patient was re-admitted with a recurrence of the cervical pulsatile mass. While the analytical study showed no elevation of inflammatory parameters (Leukocytes 8.71x10 with 47% neutrophils) and a C-reactive protein level of 8 mg/L, the CT scan showed apparent proximal migration of the stent graft with rupture at the caudal (common carotid) edge, Figure 5.
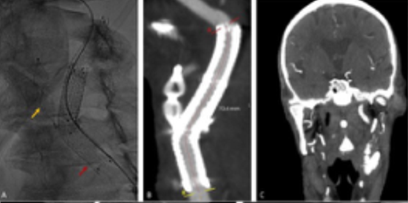
Figure 4 Post-operative imaging after external carotid artery (ECA) occlusion with a plug with an 8mm vascular plug stentgraft extension to the common carotid artery. A) Intraoperative image showing Amplatzer™ Vascular Plug 4, 8x13.5 (yellow arrow) and 8x50 Viabahn® (red arrow); B) computed tomography angiography at 24h showing ECA occlusion and patent stents with resolution of the leak; C) computed tomography angiography at one month significant reduction of the cervical hematoma (coronal view).
A) computed tomography angiography showing proximal stent graft migration and contrast leak from the distal, external carotid artery occluded with a plug (yellow arrow); B) angiogram showing contrast leak from the distal of the stent graft; C) and D) intraoperative images of the rupture exclusion through distal extension with an 8x50 stent graft.
The new rupture was corrected with an extension with an 8x50 Viabahn® stent graft through a transcervical approach. According to the previous antibiogram, the patient initiated antibiotic therapy with piperacillin-tazobactam at admission. Blood cultures were collected at admission, and they were negative after 48 hours of antibiotics. As the patient was considered high-risk for surgical approach for stent graft explant, it was decided to maintain lifelong antibiotic therapy. However, the patient evolved with nosocomial pneumonia with multiorgan failure, culminating in death on the 7th postoperative day.
Discussion
Carotid pseudoaneurysm and rupture are rare but may present as an acute life-threatening event requiring immediate management. Atherosclerosis, vasculitis, trauma, prior surgery, and infection are among the possible etiologies.1,2 Carotid blowout syndrome is also a rare entity with exceptionally high mortality, traditionally associated with head and neck cancer.3,4 Despite the etiology, which may not be apparent when a patient presents with a life-threatening event, emergent repair is mandatory to avoid neurological sequelae, hemorrhagic complications, and airway compression. However, recognition of the potential cause may dictate the approach. In this patient, despite the oncological history, there was no evidence of head and neck malignancy nor a history of previous cervical radiotherapy or surgical manipulation. The CTA showed evidence of atherosclerotic disease, which may lead to the development of an ulcerated ulcer and subsequent pseudoaneurysm. However, an infectious process must be considered in this scenario, imposing some critical considerations.
Both endovascular and open surgery are suitable approaches. Despite the lack of prospective randomized trials, recent literature showed similar short- and medium-term results when electively treating extracranial carotid aneurysms.5 However, there is even a greater lack of evidence regarding the urgent context of rupture, and literature resumes to case reports and small retrospective series.1,3,6This is well justified by the heterogeneity of patients and the frail condition they present, requiring an individualized, tailored strategy, where the best ideal solution is frequently not the most suitable approach. It is well known that prosthetic material should not be left in infected territory, which could be avoided with an open surgical approach and reconstruction with autologous venous conduit. Also, this approach would have allowed hematoma decompression and direct collection of samples for pathological and microbiological analysis. However, the risk of anastomotic dehiscence or erosion of the conduit cannot be neglected, and the exceeding morbidity and time required for bleeding control made it a less suitable option for this patient. The use of stentgrafts in carotid ruptures associated with blowout syndrome has been reported in small series with a 100% technical success rate and 12-month survival of 80%.6
In this patient, the bleeding and expansion of cervical mass were effectively controlled by this minimally invasive approach. The transcervical surgical approach, which can be performed rapidly by an experienced surgeon, facilitated the CCA access and enhanced the torqueability and navigability of the materials, avoiding potential complications of the aortic arch crossing, without adding considerable time to the procedure. Also, it would have allowed conversion to a complete surgical approach if required. There was, however, a need for a second procedure to completely exclude the leak, but this could be safely done in an elective manner without additional morbidity for the patient. The transfemoral approach in the second procedure minimized the risks of wound complications associated with surgical re-approach and enabled the extension of the stent to the CCA.
The EndoVAC technique is already considered an option for patients with carotid rupture associated with an infected prosthetic patch (recommendation class IIb, level C)7 and despite not completely applying to the present case, it would have been a helpful technique if surgical debridement was necessary, after hemodynamic stability was achieved. However, this technique is not exempted from risks related to the surgical approach,8 with potential need for subsequent reintervention and adding significant morbidity. In this patient, as the clinical evolution was favorable and there were no clinical or imaging findings suggesting macroscopic superinfection of the cervical hematoma, with dimension reduction in the control CTA, it was decided to maintain the conservative management.
The duration of the antibiotic therapy is also not well established, especially in these situations where bailout prosthetic material is left in a potentially infected territory, which cannot be subsequently removed, with some authors defending life-long antibiotic suppression.9 In this case, given the favorable course, the antibiotics were suspended after nine weeks of directed therapy, as the quinolones, the only oral antibiotic alternative, are associated with increased risk of arterial wall frailty and aneurysmal degeneration and existing evidence supports the effectiveness of a 6-week antibiotic scheme in high surgical-risk patients with infective endocarditis after transcatheter aortic valve replacement.10
The recurrence of rupture in this patient highlights the complex management of the acute phase and its implications in the subsequent course of the disease. If the most obvious conclusion to be drawn would be the recurrence of the infection, the patient did not present any signs of systemic inflammatory response, and the blood cultures taken at the admission of the new event, more than two months after the cessation of antibiotic therapy, remained negative. This raises the question of whether the prolonged antibiotic therapy could have prevented this recurrence. On the other hand, the imaging study suggests a stent migration with a recurrence of the rupture at the distal edge, possibly indicating a potential technical failure. However, the rupture's topography also differs from the initial episode, which occurred near the carotid bifurcation. Could the progression of the infection have led to additional wall destruction culminating in a new rupture point and the dehiscence of the stent-graft? Could this arterial fragility have progressed despite the control of the infectious focus? Could an open reconstruction surgery have been more appropriate? Or would it have turned into a nightmare where arterial fragility made any anastomosis unfeasible? All these questions remain unanswered, but they should cross the mind of any surgeon dealing with vascular emergencies.
Conclusion
This report underscores the complexities involved in managing carotid ruptures and pseudoaneurysms. Endovascular therapy offers a potential reduction in the morbidity associated with open surgical repair, presenting itself as a viable short- and mid-term strategy, particularly for frail patients. However, the implications of each treatment option must be considered over the entire follow-up period, considering the patient's life expectancy. This adds a layer of complexity to the decision-making process, requiring a careful balance between the favorable short-term outcomes of palliative endovascular treatment and the potential medium- and long-term complications.