Introduction
Superior vena cava syndrome (SVCS) is a group of signs and symptoms caused by blood flow obstruction through the superior vena cava (SVC) and/or innominate veins.1,2 Common symptoms include cough, dyspnea, and orthopnea. The most frequent signs are facial swelling and redness (plethora), swollen upper limbs, and enlarged veins in the chest wall and neck.1,2
Both malignant and non-malignant conditions can cause blockage of the SVC. In cancer-related SVCS, the blockage can be caused by intraluminal tumor cell invasion, external compression, or thrombosis.1
Mediastinal malignancies, particularly lung cancer, cause most cases of SVCS. This includes up to 10% of people with small cell lung cancer and 2-4% of all lung cancer patients. Non-Hodgkin lymphoma and metastatic tumors are also common culprits. SVCS can be the first sign of a previously unknown cancer in up to 60% of patients, and therefore, an accurate diagnosis evaluation is essential before any emergency treatment is given.1
There is no universally accepted, evidence-based approach to treating SVCS, but the treatment has two main goals: relieving symptoms caused by the blockage and addressing the underlying illness. Options for treating the obstruction include radiation therapy, chemotherapy, open surgery, and endovenous procedures.1 In critical situations where breathing or circulation are compromised, immediate airway stabilization, breathing support, and venous recanalization are paramount.1,2 Endovascular intervention with venous stenting as the primary treatment option has recently gained traction due to its numerous benefits: (1) high success rate and a low likelihood of complications; (2) rapid symptom relief, often within 24 to 72 hours; (3) noninterference with subsequent necessary treatments for the underlying cancer such as chemotherapy or radiation therapy; and (4) the procedure can be performed without definitive histologic diagnosis, offering greater flexibility in patient management.3
There is a debate surrounding the use of covered versus uncovered, or bare metal stents (BMS) for improved technical success and stent patency. Although significant differences in technical success rates between stents have not been shown, retrospective studies have suggested that covered stents provide better long-term permeability compared to BMS.4
However, covered stents can obstruct important collateral veins or even the opposite brachiocephalic vein. They also may not be readily available in larger sizes and require a larger sheath size than BMS. It is also noteworthy that balloon-expandable stents, unlike self-expanding ones, can migrate after tumor shrinkage, requiring caution in specific clinical contexts.3,4
Aim
This study aims to provide a comprehensive analysis of the existing literature concerning the outcomes of covered stents versus BMS, focusing on technical and clinical success and to the best of our knowledge, no past review has directly attained this dichotomy in malignant SVCS.
Methods
This systematic review and meta-analysis were conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.5
Literature search
Two authors (FB, APC) searched the COCHRANE and PUBMED online databases to identify articles presenting the outcomes of endovascular stenting using covered stents or BMS, in treating SVCS caused by intra-thoracic malignancies. The search utilized the query: "(superior vena cava syndrome) AND (Malignant) AND (Endovascular treatment)”, in the last 11 years (2013-2024). Initial screening of retrieved studies involved reviewing titles and abstracts, followed by a thorough examination of the full texts of the screened studies by both authors to assess eligibility for inclusion. In addition to the initial search, the references of relevant systematic reviews were systematically scrutinized to identify potentially relevant additional papers (backward citation). The final search was performed on February 5, 2024.
Study selection
Two authors (FB, AC) independently carried out the process of study selection. The criteria for selection were as follows: (1) Full text in English, Portuguese, or Spanish. (2) Original studies including 10 or more adult human patients. (3) In cases where studies addressed interventions in the SVC along with other vessels, studies providing discernible data on technical and clinical outcomes of SVC interventions, with or without the participation of brachiocephalic veins, were considered for inclusion. (4) Focus on cases where the cause of SVCS is confirmed to be malignant. (5) The outcomes must be referred to the stent implantation, without other endovascular devices associated.
Data collection and quality assessment
The data extracted from each incorporated study encompassed the following categories: (1) study details, including the first author and study design (prospective/retrospective); (2) demographic information about the study population, such as size, mean age, and sex distribution; (3) procedural details, involving stent type and manufacturer, technical and clinical success rates, complications, post-procedural medication; and (4) follow-up data, comprising primary and secondary patencies, symptom recurrence, re-interventions, and survival. Two authors (FB, APC) independently conducted the data extraction. In instances of disagreement between reviewers, resolution occurred through discussion, and when necessary, consensus was reached with a third author (RG). The methodological quality of the included studies was evaluated for the risk of bias using the Newcastle-Ottawa scale.6
Outcomes
Primary outcomes were defined as technical and clinical success. Additionally, secondary outcomes were defined as primary and secondary patency, complications, recurrence of symptoms, reinterventions, and mean survival.
Statistical analysis
The software Review Manager 5.4 (REVMAN) was used to analyze data. Pooled prevalence was presented as a percentage (%) with 95% confidence interval (CI) for categorical variables and as a mean and standard deviation (SD) for continuous variables. Direct comparison of both techniques was calculated when possible. Odds ratios (OR) and 95% CI were used for dichotomous variables, and mean differences (MDs) with 95% CI for continuous data.
Statistical heterogeneity, defined as a measure of the variability of outcomes between studies, was assessed by the Cochran´s Q test: H2 test (Higgins and Thompson) was used to quantify the magnitude of heterogeneity. The parameter I2 retrieved from the H2 test was used with a cut-off of 25% for low, 25-50% for intermediate, and above 50% for high heterogeneity. A fixed-effects model was used when heterogeneity (I2) was less than 50%, and a random effects model was used when heterogeneity (I2) was high.
Results
Study selection
The initial search yielded 74 studies, Figure 1. After removing duplicates and the screening process based on title and abstract, 22 studies were thoroughly examined in full text. One additional study was included by backward citation. After full text analysis, 17 studies fulfilled all the inclusion criteria, encompassing a total of 1123 patients. Subsequent steps involved data extraction and assessment of study quality. Common reasons for exclusion included inadequate sample sizes or unclear reporting of outcomes related to the Superior Vena Cava (SVC), abstract or full text not available, SVCS caused by a benign cause, and the evaluation of outcomes regarding the implantation of stent and other endovascular devices at the same time.
Patient demographics and study characteristics
The aggregate number of reported patients amounted to 1123. The average age of the entire patient cohort was 64.8 years, and 76.5% were male (95% CI 69.9-83.1).
A comprehensive overview of the characteristics of the included studies is presented in
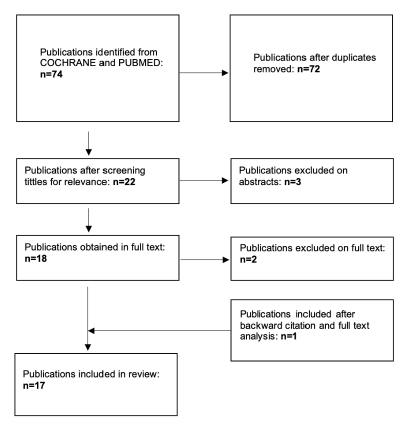
Figure 1 PRISMA flowchart showing selection of studies comparing covered with uncovered stents for the treatment of malignant superior vena cava syndrome.
Table 1 Characteristics of the 17 studies presenting outcomes of endovascular stenting using covered or bare metal stents, in treating superior vena cava syndrome caused by intra-thoracic malignancies, included in the systematic review.
| Author | Type of stent | N | Mean age (years) | Male/Female | Technical success (%) | Clinical Success (%) | Stents Details | Post procedural medication | Mean survival | Follow up protocol |
|---|---|---|---|---|---|---|---|---|---|---|
| Andersen et al.(7) | BMS | 12 | 69 | 5/7 | 75 | 92 | Zilver Vena | Non specified Anticoagulation and aspirin | 2 M (1-5M) | Mean FU: 2.5M (1-6) Clinical: patient report Imaging: CT at 1-3M |
| Wei et al.(8) | BMS | 16 | NR | 14/2 | 100 | 100 | Wallstent | Non specified Anticoagulation | 7M (1-18) | Mean FU: NR Clinical: patient reported Imaging: NR |
| Kuo et al.(9) | BMS | 12 | 58.4 | 12/0 | 100 | 100 | Wallstent | Warfarin if SVC with significant thrombus, and clopidogrel | NR | Mean FU: 11.5M (0.3-17) Clinical: patient report Imaging: CT at 3 and 6M, and annually |
| Leung et al.(10) | BMS | 56 | 64 | 40/16 | 97 | 93 | Wallstent | Non specified anticoagulation | 199d (2-1156) | Mean FU: NR Clinical: patient report Imaging: NR |
| Juscafresa et al. (11) | BMS | 33 | 57.6 | 23/10 | 100 | 84.8 | WallStent, Protégé or Express | Non specified anticoagulation | 13M | Mean FU: NR Clinical: patient report Imaging: NR |
| Matthaiou et al.(12) | BMS | 156 | 62 | 132/34 | 99.3 | 96.7 | E-luminexx, Protégé, Sinus XL | NR | NR | Mean FU: 320d (1-1795) Clinical: patient report Imaging: CT at 3 and 6M, and annually |
| Guerrero-Macías et al.(13) | BMS | 54 | 56.1 | 31/23 | 94.4 | NR | SMART | Anticoagulation if SVC with significant thrombus | 2.4M (CI 95%: 1.28-4.80) | Mean FU: 14.3M Clinical: patient report Imaging: NR |
| Liu et al. (14) | CS | 32 | 57 | 25/7 | 100 | 100 | Fluency plus | warfarin | NR | Mean FU: 6.5M (1.5-24) Clinical: patient report |
| BMS | E-luminexx, Wallstent | Imaging: NR | ||||||||
| Bustgens et al.(15) | BMS | 141 | 64.6 | 86/55 | 97.9 | 96.45 | SMART, Wallstent, Zilver Vena, Epic, Unspecified | Non specified anticoagulation | 101d | Mean FU: NR Clinical: patient report Imaging: NR |
| Irace et al.(16) | BMS | 42 | 72 | 36/6 | 100 | 98 | Memotherm, Wallstent | Warfarin for at least 6M; and aspirin | NR | Mean FU: 14M (1-28) Clinical: up every 4M with physical examination Imaging: CT annually |
| Xu et al.(17) | CS | 2 | 59.5 | 2/0 | 100 | 100 | Viahbahn | In-hospital 12000 IU of heparin up every 12h | NR | Mean FU: 18M (12-24) Clinical: patient report Imaging: NR |
| BMS | 8 | 59.6 | 6/2 | 100 | 100 | SMART and Wallstent | Mean FU: 14.4M (1-36) Clinical: patient report Imaging: NR | |||
| Talens et al.(18) | BMS | 114 | 61.18 | 103/11 | 99.1 | 85.6 | WallStent, Symphony, Expander, Absolute; Luminexx, Protégé, Epic | At least 6M of acenocoumarin or aspirin | 210d (9-1053) | Mean FU: NR Clinical: patient report Imaging: NR |
| Chan et al.(19) | BMS | 104 | 65 | 81/23 | NR | NR | Wallstent | Non specified anticoagulation and antiagregation | 8.4M | Mean FU: 2M Clinical: patient report Imaging: NR |
| Fagedet et al.(20) | BMS | 164 | 59.9 | 123/41 | NR | 84.5 | Wallstent, Memotherm, SMART, Strecker, Protégé | Non specified anticoagulation in particular cases; and 3-6M of aspirin | NR | Mean FU: 355.2d Clinical: patient report Imaging: CT every 6M for 1y and annually |
| Gwon et al.(21) | CS | 37 | 60.3 | 33/4 | 100 | 95 | ComviStent | Continuous infusion of heparin (500 U/h) for 2-5 days. Subsequently, administration warfarin or aspirin for at least 3 months. | 141d (CI 95%: 81-201) | Mean FU: 150d (4-856) Clinical: patient report Imaging: CT at 1 and 6M |
| BMS | 36 | 62.3 | 31/5 | 100 | 92 | Zilver Vena | 100d (CI 95%: 137-189) | |||
| Cho et al.(22) | CS | 40 | 61.4 | 35/5 | 100 | 92 | ComviStent | Non specified anticoagulation | 163d (CI 95%: 137-189) | Mean FU: 175d (3-873) Clinical: patient report Imaging: NR |
| Wang et al.(23) | CS | 30 | 61.6 | 23/7 | 100 | 100 | Fluency | 3 days using low-molecular-weight heparin (6000 IU/12 h) after which they received oral warfarin. | 175d | Mean FU: 6.2M (0.3 - 14) Clinical: patient report Imaging: CT at 1, 3 and up every 6M |
| BMS | 34 | 61.2 | 18/16 | 100 | 100 | Luminexx | 159d |
BMS - Bare metal stent; CS - Covered stent; FU - Follow-up; CT - Computed tomography; NR - Not reported.
Table 2 Risk of bias according to the Newcastle-Ottawa scale
| Study | Selection (S) | Comparability (C) | Exposure/ Outcome (E/O) | Quality Total (max=9) | |||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | E/O1 | E/O2 | E/O3 | ||
| Andersen et al.(7) | * | * | * | * | 4 | ||||
| Wei et al.(8) | * | * | * | * | 4 | ||||
| Kuo et al.(9) | * | * | * | * | 4 | ||||
| Leung et al.(10) | * | * | * | * | ** | * | * | * | 9 |
| Juscafresa et al.(11) | * | * | * | * | 4 | ||||
| Matthaiou et al.(12) | * | * | * | * | 4 | ||||
| Guerrero-Macías et al.(13) | * | * | * | * | 4 | ||||
| Liu et al.(14) | * | * | * | * | 4 | ||||
| Bustgens et al.(15) | * | * | * | * | 4 | ||||
| Irace et al. (16) | * | * | * | * | 4 | ||||
| Xu et al.(17) | * | * | * | * | 4 | ||||
| Talens et al. (18) | * | * | * | * | * | * | 6 | ||
| Chan et al.(19) | * | * | * | * | 4 | ||||
| Fagedet et al. (20) | * | * | * | * | 4 | ||||
| Gwon et al.(21) | * | * | * | * | ** | * | * | * | 9 |
| Cho et al.(22) | * | * | * | * | 4 | ||||
| Wang et al.(23) | * | * | * | * | ** | * | * | * | 9 |
Among the 17 included studies, 15 were retrospective, one was prospective, and one had both prospective and retrospective arms. No randomized controlled trials or multicenter studies were included. The evaluation of bias risk, conducted using the Newcastle-Ottawa scale, is detailed in Table 2, with all papers scoring between 4 and 9, most of them classified as poor quality.
All studies employed clinical criteria to determine the need for intervention, coupled with pre-operative imaging to characterize the nature of the obstruction.
The follow-up procedures were not consistent across the studies. When outlined beforehand, they involved regular imaging and clinical assessment in 7 out of 17 studies. The remaining studies relied on patients reporting their own symptoms. The duration of follow-up varied, with mean lengths ranging from 2 to 18 months. Importantly, all studies endeavored to follow up with patients until death, the designated study endpoint, or to the date they’ve lost follow up.
Stent type
The details regarding the type of stent employed were documented in all studies, encompassing a total of 1123 patients. Uncovered stents were the exclusive choice in 12 studies. A solitary study opted for covered stents alone,22 while 4 studies employed both covered and uncovered stents.14,17,21,23 The most common uncovered stent used was Wallstent™Endoprosthesis (Boston Scientific, Maple Grove, MN, USA), and in the covered group the Fluency™Endovascular Stentgraft (Bard, Murray Hill, NJ, USA) and Vascular Co mVI (TaeWoong Medical, Gimpo, Korea) Stents.
The rationale for covered stents in these studies stemmed from the anecdotal reports of tumor ingrowth in uncovered stents and the risk of iatrogenic injury to the SVC. However, there is no unanimous agreement on whether covered or uncovered stents yield superior outcomes.14,17,21,23) Overall, 1014 (90.3%) and 109 (9.7%) received uncovered or covered stents, respectively.
Technical success
Across all comparative studies,17,21,23 the technical success rate for both covered and uncovered stents was 100%, and accordingly, the comparative Odds Ratio (OR) could not be calculated. The pooled data for covered stents shows a technical success rate 100% (CI not estimable [NE]). The pooled data for uncovered stents was 97.9% (95% CI 96.5-99.3).
Only two studies have not reported technical success.19,20 Most studies have defined technical success as the successful navigation and deployment of the stent across the obstruction or narrowing, coupled with evidence of restored flow on post-intervention venography. Further criteria for technical success were specified, including achieving a final pressure gradient below 10 mmHg in two studies21,22 and less than 50% residual stenosis in one study.7
Clinical success
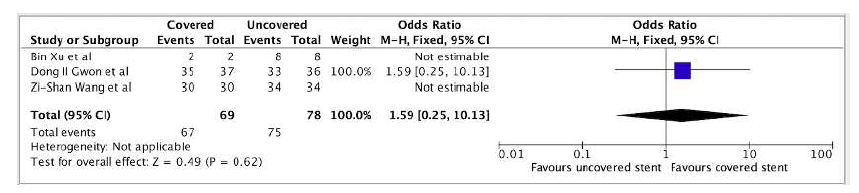
One comparative study21 demonstrated an OR of 1.59 (95% CI 0.25-10.13) favoring covered stents without reaching statistical significance, Figure 2. Pooled data from additional studies revealed clinical success rates of 95.4% (95% CI 89.2-100) and 92.0% (95% CI 87.0-97.0) for covered and uncovered stents, respectively.
All but two studies defined clinical success as an immediate improvement in symptoms, whether partial or complete, assessed either through patient-reported symptoms or the Kishi scoring system.24 Dyspnea was excluded from consideration as a symptom assessed for defining clinical improvement in two studies,21,22 given its common occurrence in underlying pulmonary conditions and its frequent association with tumor invasion into the bronchus or pulmonary vessels. One study did not report clinical success,19 and the remaining study characterized clinical success as a pressure gradient of <10 mmHg after stent post-insertion.23
Complications
Complications were described in 8/17 studies, all of which assessed uncovered stents. Pooled data analysis revealed that uncovered stents had 6.2% (95% CI: 0.6-11.8) rate of complications, while covered stents had 0% (95% CI: NE). The CIRSE classification system25 was used to discriminate complications as listed in Table 3.
Eight patients died in the first 24 hours, with cardiac tamponade caused by SVC rupture being the most common cause of death. The most common serious complication (above Grade 3) was bleeding while on long-term anticoagulation. The most common complications below Grade 3 were stent migration and hematoma at the puncture site, Table 3.
Table 3 Minor and major complications by Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 6 |
|---|---|---|---|---|
| Puncture site hematoma (N=5) | Stent Migration (N=8) | Cardiac tamponade due to iatrogenic SVC perforation (N=3) | Bleeding event on anticoagulation (N=14) | Mortality in 24h - Cardiac Tamponade (3), major bleeding (1), RI (1), HF (1), Hemopericardium (1), Hemoperituneum (1) |
| Fever (N=1) | Arrythmia - VT (N=1) | Haemopericardium (N=1) | Pulmonary embolism/ DVT (N=1) | |
| Bleeding event due to CDT (N=1) | Puncture site infection (N=2) | |||
| Arterial injury (N=1) | ||||
| Pulmonary oedema (N=5) |
CDT - Catheter-Direct thrombolysis; VT - ventricular Tachycardia; SVC - superior vena cava; DVT - deep venous thrombosis; HF - heart failure; RI - respiratory insufficiency
Patency and Re-interventions
Primary patency denoted a stent remaining open without additional procedures throughout the study period. Conversely, secondary patency encompassed stents that required further interventions to maintain patency. Nine out of seventeen studies8,11,12,14-16,20-22 including 651 patients (614 treated with BMS and 37 with covered stents), reported primary patency. In contrast, secondary patency was reported only in one study,11 with 33 patients treated with BMS.
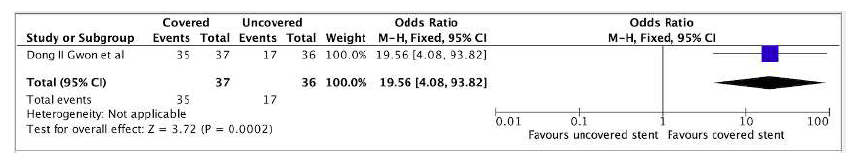
A single study directly comparing covered stents and BMS provided data on primary patency at 1, 3, 6, and 12 months.21At 1 and 3 months, no significant differences were observed between the two stent types: OR 1.03 (95% CI 0.06-17.09), and OR 5.00 (95% CI 0.98-25.45), respectively. However, at 6 months, covered stents demonstrated a statistically significant advantage in primary patency compared to BMS (OR 8.75, 95% CI 1.79-42.67). This advantage became even more pronounced at 12 months, with covered stents exhibiting a nearly twofold increase in primary patency compared to BMS (OR 19.56, 95% CI 4.08-93.82) (Figure 3).
Pooled data from studies at 12 months further reinforced the suggested superiority of covered stents in maintaining vessel patency. Covered stents exhibited a primary patency rate of 90.9% (95% CI 45.9-100), compared to 77.1% (95% CI 46.0-100) for BMS. A single study reported a secondary patency rate of 97% at 12 months for BMS.11
Recurrence of symptoms during follow-up was documented in eleven out of seventeen studies, encompassing a total of 132 patients, at a median time range of 48 hours to 18 months.9,10,13-16,18,20-23 The symptoms recurred in 7 patients treated with covered stents. The primary reasons for recurrence in both groups were intra-stent thrombosis, tumor overgrowth and compression, or tumor ingrowth through the stent.
Pooled data from studies revealed a difference in re-intervention rates between covered stents and BMS, with covered stents exhibiting a lower re-intervention rate of 1.7% (95% CI 0.0-28.2) compared to 9.0% (95% CI 2.7-15.4) for BMS. Three out of seventeen studies did not describe any re-interventions.8,13,17 Re-intervention procedures included balloon dilatation, thrombolysis, thrombectomy, and additional stenting.
Survival
Data on mean survival were reported in ten (including two comparing covered stents with BMS, and one describing only covered stents) out of the seventeen studies included in this analysis.7,8,10,11,13,15,19,21-23 The average mean survival across studies was 5.7 months, ranging from 2 to 13 months.
One study reporting only BMS observed a significant association between longer survival and stenting as a first-line intervention compared to salvage stenting.10 Additionally, another study, also including only BMS, reported significantly improved survival in patients with a distal attachment in the SVC or the right innominate vein compared to those with an attachment in the right jugular vein, left jugular vein, or left innominate vein probably due to longer and more severe obstructions, as well as less possibility of collateral circulation.13
Post-procedural antithrombotic management
All but the study of Matthaiou et al12 documented the use of long-term anticoagulation and/or antiplatelet therapy. However, the specific anticoagulation regimens employed varied significantly in terms of medication type and treatment duration.
Among the studies evaluating BMS that specified their regimens, one study utilized six months of warfarin and aspirin treatment,16 while another study employed six months of acenocoumarin or aspirin.18
Gwon et al. defined warfarin or aspirin for at least 3 months for covered stents and BMS.21 The types of anticoagulation/antiplatelet medications used included warfarin, aspirin, heparin, antiplatelet drugs, and various combinations of these medications.
Discussion
In malignant SVCS, SVS's low pressure and cancer-induced hypercoagulable state make it prone to both tumor compression and thrombus formation, leading to critical conditions that may need emergent intervention for symptom relief immediately.1 There is currently no consensus on what type of stent to use in managing SVCS to confirm an evidence-based practice.
This comprehensive review and meta-analysis confirmed the effectiveness and safety of both types of stents, covered and uncovered, for malignant SVCS. The findings demonstrate high technical and clinical success rates of 100% VS 97.9%, and 95.4% VS 92%, respectively, in alignment with the existing literature.1
Additionally, patency rates were 90% for covered stents and 77.1% for BMS in the first year following the procedure, emphasizing the long-term efficacy of covered stents. Covered stents presented with 0% complication rates vs. 6.2% in BMS, but this was probably due to publication bias. Regarding reintervention rate, it was also lower, with 1.7% vs. 9% favoring the covered stents group.
The differences in patency and reintervention were probably due to ingrowth of the tumour. Gwon et al. found no cases of tumor ingrowth in the covered stent group. In contrast, stent occlusion caused by tumor ingrowth was observed in seven of the ten patients in the BMS group with associated complications.21 In another study, comparing both types of stents Wang et al. also found no evidence of tumor ingrowth in patients treated with covered stents. In contrast, four of six patients with stent dysfunction in the BMS group suffered from tumor ingrowth.23
Some complications, such as stent migration, are described in the literature. This long-term complication, occasionally fatal, remains a risk and is potentially prevented using a specific design of stent, partially covered with bare extensions and an outer bare stent. Additionally, using a slightly oversized stent (10-20%) might help prevent migration.21-23
A second described complication is contralateral vein occlusion. Placing a long-covered stent across the brachiocephalic vein confluence could potentially block the contralateral vein, leading to upper extremity vein thrombosis. This complication was not observed in any study using covered stents,21-23, suggesting that unilateral obstruction relief might allow for sufficient collateral flow through the contralateral vein via the cervical and intracranial routes.
The main complication described in this analysis for the BMS was stent migration. BMS, particularly Wallstent, exhibit a tendency to migrate if not carefully centered within the stenosis or if their diameter is not appropriately selected.18 The stent diameter should exceed the diameter of the target vein by 15-20%.18
Despite increasing evidence supporting stenting, a standardized approach to anticoagulation for SVCS, both during the procedure and in follow-up, remains elusive.1,2,4 There is no conclusive evidence that anticoagulation necessarily leads to better outcomes.26 Given that adverse events associated with anticoagulation represent a significant portion of post-procedure complications, further research is needed to determine whether anticoagulation is truly necessary and, if so, to identify the optimal regimen.
The main limitations to this review lie in the absence of randomized controlled trials or well-designed prospective studies that clearly defined follow-up protocols, and the lack of studies directly comparing patients treated with covered VS uncovered stents.
The majority of included studies were retrospective and conducted at single centers, raising concerns about potential selection or publication bias and the possibility of overestimating the reported outcomes. Furthermore, definitions of outcomes like technical and clinical success and primary and secondary patency varied significantly across studies, hindering the utility of a meta-analysis.
Another limitation of our study is the exclusion of a sub-analysis comparing dedicated venous stents with non-dedicated venous stents, as only Matthaiou et al. addressed this.12 Previous studies have highlighted the limitations of stainless-steel stents, such as the Gianturco Z stent and Wallstent, in terms of rigidity and foreshortening, respectively. In contrast, nitinol stents avoid these issues and appear more suitable for treating malignant SVCS.12
Given these considerations, the exclusion of this comparison constitutes a methodological bias in our study, as it would have offered critical insights into the use of dedicated venous stents.
Moreover, this omission underscores a significant scientific gap that warrants further investigation.
Conclusion
This review and meta-analysis confirm the effectiveness and safety of covered and uncovered stents for malignant SVCS. Our findings also suggest that endovascular placement of covered stents represents a promising approach with superior primary patency rates (versus BMS). However, limited data from heterogeneous studies hinders definite conclusions. Further research of higher methodological quality is crucial to elucidate the full extent of covered vs. uncovered stenting efficacy.