INTRODUCTION
Allergic contact dermatitis (ACD) to the glucose sensor FreeStyle Libre® has been increasingly recognized in the past few years. The allergen responsible for most cases is isobornyl acrylate (IBOA; CAS 5888-33-5), a substance present within the sensor that migrates through the adhesive, thereby reaching the skin.1 Herein we report a case of leukoderma induced by FreeStyle Libre®, a rare phenomenon occurring along with ACD to this medical device.
CASE REPORT
A 41-year-old woman with type 1 diabetes mellitus was referred to our department due to suspicion of contact dermatitis to the glucose sensor FreeStyle Libre®, which she had been using for 6 months. Besides the dermatitis itself, the patient also presented several hypopigmented macules and patches on the outer side of both arms, corresponding to the areas where the sensor had been applied (Fig.1A). She denied any personal or familiar history of vitiligo or atopy, and there were no similar lesions in the rest of the skin. She had not used other glucose sensors or insulin pumps and she also denied application of corticosteroids or other depigmenting agents.
Patch testing was performed with the Portuguese baseline series, acrylates series and plastic & glues series (Chemotechnique Diagnostics, Vellinge, Sweden), and also with a piece of the adhesive patch of the glucose sensor “as is”, with methylhydroquinone 1.0% in pet., hydroquinone monobenzylether 1.0% pet. and with IBOA (purchased from Sigma-Aldrich and diluted 0.1% in pet. by the hospital pharmacy).
Patch tests were performed on the upper back using IQ Ultra test chambers (Chemotechnique Diagnostics). Readings were performed on day 2 (D2) and D4, and patch test reactions were classified according to ESCD criteria.2 A biopsy of one of the hypopigmented areas was concurrently undertaken.
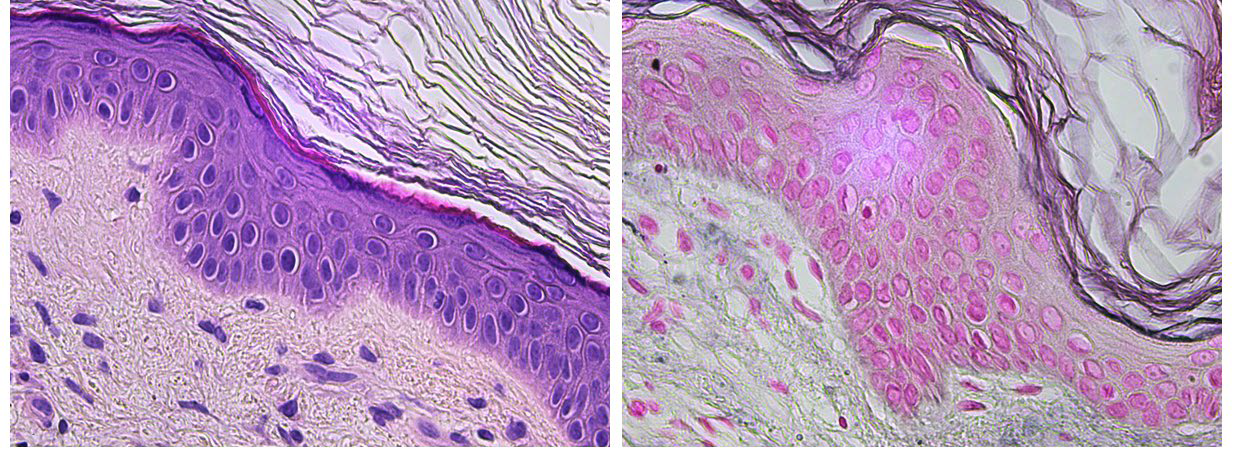
Positive reactions on patch tests were observed only for IBOA (++), with no reaction to the fragment of the tape of the glucose sensor (Fig. 1B). Histopathology of a hypopigmented area showed a complete absence of epidermal melanocytes (Fig. 2A), which was confirmed by Fontana
Masson staining (Fig. 2B). The patient stopped using the sensor due to the cutaneous side effects, with a significant impact both in quality of life and in glycemic control. After 6 months of follow-up, she remains clinically stable, with no new lesions and awaiting transition to a new glucose sensor.
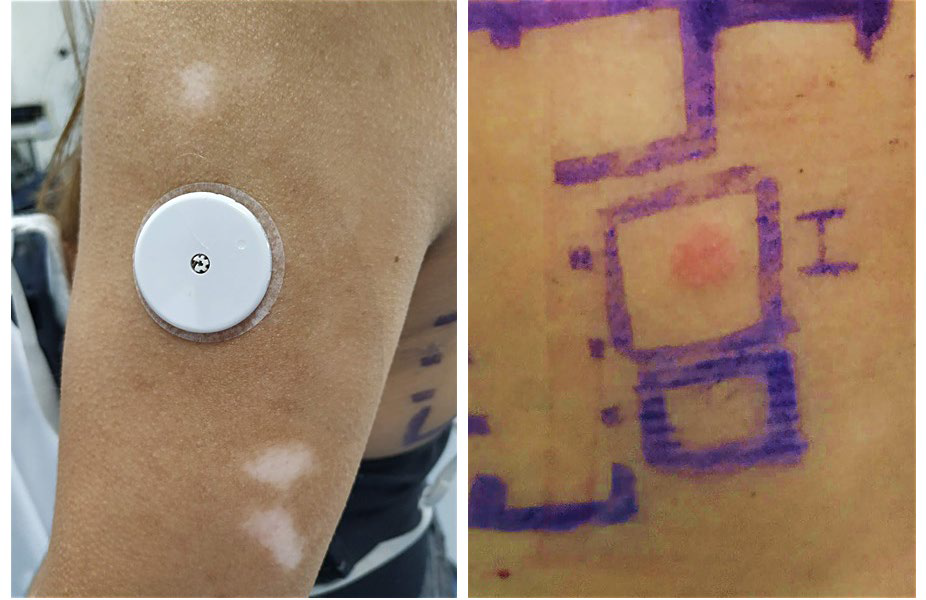
Figuras 1 Leukoderma after ACD to the glucose sensor FreeStyle Libre A; (A) - clinical picture showing hypopigmented macules and patches on the outer side of the left arm; (B) - patch tests confirmed ACD by revealing a positive reaction (++) to IBOA on the first reading (D2).
DISCUSSION
ACD to medical devices, including FreeStyle Libre®, is an increasingly recognized problem, posing a great challenge not only to patients suffering from this condition but also to clinicians dealing with them.3
Nonetheless, to our knowledge, only one case of acquired leukoderma following sensitization to FreeStyle Libre® has been reported in the literature.4
Acquired leukoderma may occur in an area previously affected by ACD, and has been described in several medical devices with adhesives.5 Regarding FreeStyle Libre®, IBOA does not seem to be the culprit substance, as it is not a recognized depigmenting agent.1Moreover, the occurrence of leukoderma along with ACD to FreeStyle Libre® is rare, arising the suspicion for other explanation beyond IBOA. In fact, it is hypothized that hydroquinone monomethyl ether (HMME) may be the responsible for this reaction.4HMME is a known depigmenting agent, and has even been responsible for cases of occupational leukoderma.6In this scenario, it is thought that HMME may have a direct toxic effect on melanocytes, potentially resulting in subclinical inflammation via melanocyte destruction.4 This explains the histopathological findings in our patient, notably the absence of epidermal melanocytes.
Actually, HMME has been identified along with the IBOA in the sensor, where it acts as an inhibitor to prevent inadvertent IBOA polymerization. It is known that IBOA is not present in the adhesive part of FreeStyle Libre®; however, it originates from the plastic material of the sensor itself and subsequently diffuses through the plaster to the skin, a phenomenon probably added by occlusion and sweating.7-9
Therefore, it is assumed that, in some instances, HMME may also migrate through the adhesive along with IBOA, resulting in a phenomenon of ACD with associated leukoderma, as in our patient.
Although rare, acquired leukoderma following ACD to FreeStyle Libre® can occur. Therefore, clinicians dealing with these patients should be alert to this possibility.