Introduction
A novel virus of the Coronaviridae family, designated “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2), was identified in Wuhan, China, in December 2019. The infection caused by SARS-CoV-2, also known as COronaVIrus Disease-19 (COVID-19), has rapidly spread throughout the world, becoming pandemic in early March 2020, with significant health, social, and economic impact.1 To date, more than 55.6 million people have been infected by SARS-CoV-2 worldwide.2
The clinical spectrum of COVID-19 is heterogeneous, ranging from asymptomatic or mild symptoms to severe and fatal illness. Over the course of the disease, diverse organs and systems can be affected. In the skin, several clinical manifestations have been associated with COVID-19. Urticarial, morbilliform, and varicelliform eruptions, pernio-like lesions, purpuric lesions, acro-ischemic lesions, and retiform purpura, may occur early and their identification may be relevant for the initial diagnosis of SARS-CoV-2 infection.1,3-7However, delayed dermatological manifestations of the infection can also hypothetically be expected, similar to what is observed with other viral diseases.
The hair cycle is especially susceptible to endogenous and exogenous stimuli, including febrile states and emotional stress,8-11which are a constant in this pandemic era.12
In this case series, we describe the clinical characteristics of patients with a recent history of SARS-CoV-2 infection who presented in our Hair Consultation due to acute telogen effluvium (TE). Our objectives were to assess the timing and duration of the hair loss and the presence of scalp symptoms after the diagnosis of SARS-CoV-2 infection.
Material and Methods
We performed a registry of patients with laboratory-confirmed SARS-CoV-2 infection who developed acute TE and actively sought medical care at the Hair Unit of Dermatology Center of CUF Descobertas Hospital, from March 2020 to October 2020. Patient demographics, the month of SARS-CoV-2 infection diagnosis by polymerase chain reaction of nasopharyngeal swab test, COVID-19 associated symptoms, latency and duration of hair loss, associated scalp symptoms, and local of hair loss (scalp, body) were recorded. The anonymized patient data were analyzed using SPSS Statistics, 23rd version.
Results
From March 2020, we observed 27 patients with acute TE following SARS-CoV-2 infection. The mean age was 45 (( 16) years. There was a female preponderance with a female to male ratio of 6.75:1.
Of the 27 patients with acute TE, 5 (18.5%) patients also referred trichodynia. The median time of latency of increased hair loss since SARS-CoV-2 infection diagnosis was 10 weeks. In a third of cases (n=9, 33.3%), hair loss occurred early (with a latency of 3 weeks or less).
Overall, the clinical picture of acute TE was characterized by diffuse hair loss with a bitemporal recession of the hairline. The pull test was highly and diffusely positive. Trichoscopy revealed empty follicles and numerous short regrowing hairs of normal thickness. No signs of peripilar inflammation were observed. In 3 (11%) patients, hair loss involved not only the scalp but also the body. TE resolution was documented in 16 (59%) cases with a median duration of hair loss of 24.5 days.
Regarding SARS-CoV-2 infection, the great majority of patients (n=23, 85%) had symptoms and 4 (15%) patients were asymptomatic. The most common symptoms were fever (n=17, 63%), ageusia (n=8, 30%), cough (n=6, 22%), myalgia (n=5, 18.5%), anosmia (n=4, 15%), and thoracalgia (n=3, 11%). Two patients also reported cutaneous manifestations, such as pernio-like lesions (n=1) and maculopapular eruption (n=1). Most symptomatic cases of COVID-19 were mild in severity and only 3 (11%) patients required hospitalization. None of them needed admission to the intensive care unit.
Figures 1, 2 and 3 illustrate the clinical and trichoscopic features of one of the patients with acute TE following SARS-CoV-2 infection.
Patients' data are summarized in Table 1.
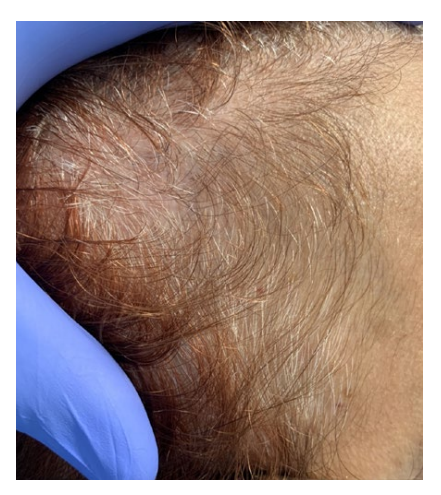
Figure 1 Acute telogen effluvium after SARS-CoV-2 infection diagnosis. Clinical picture showing temporal recession.
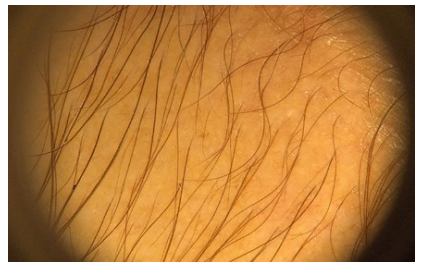
Figure 2 Acute telogen effluvium after SARS-CoV-2 infection diagnosis. Trichoscopy showing decreased hair density with the presence of empty follicles and short regrowing hairs.

Figure 3 Acute telogen effluvium after SARS-CoV-2 infection diagnosis. Amount of hair shed on a wash day showing stage 6 of hair loss severity (about 750 hairs).
Table 1 Summary of patients with acute telogen effluvium after SARS-CoV-2 infection diagnosis.
| Patient | Gender | Age | SARS-CoV-2 infection | Trichodynia | Telogen effluvium | ||||
|---|---|---|---|---|---|---|---|---|---|
| Month of diagnosis | Systemic symptoms | Latency period (weeks) | Local of hair loss | Resolution | Duration of hair loss (days) | ||||
| 1 | M | 45 | March | Fever, thoracalgia | Yes (pain) | 9 | Scalp | Yes | 8 |
| 2 | F | 60 | March | Fever, cough, headache, diarrhea | Yes (burning sensation) | 11 | Scalp | Yes | 10 |
| 3 | F | 41 | March | No | No | 11 | Scalp | Still active | - |
| 4 | F | 18 | Mar ch | A geusia | No | 14 | Scalp | Yes | 28 |
| 5 | F | 31 | April | Fever, cough, anosmia | No | 13 | Scalp and body | Yes | 56 |
| 6 | F | 63 | April | No | No | 10 | Scalp | Yes | 84 |
| 7 | F | 50 | April | Cough, ageusia | No | 3 | Scalp | Yes | 21 |
| 8 | F | 53 | April | Fever, myalgia | No | 12 | Scalp | Yes | 21 |
| 9 | F | 39 | April | Fever | No | 14 | Scalp | Yes | 28 |
| 10 | F | 26 | April | Fever, anosmia, ageusia, pernio-like lesions | No | 11 | Scalp | Yes | 35 |
| 11 | F | 58 | April | No | No | 11 | Scalp | Yes | 21 |
| 12 | F | 24 | April | Fever, cough, ageusia | No | 5 | Scalp | Still active | - |
| 13 | F | 23 | April | Ageusia | No | 12 | Scalp | Still active | - |
| 14 | M | 48 | April | Fever, thoracalgia, myalgia | No | 12 | Scalp | Yes | 90 |
| 15 | M | 26 | May | Fever, maculopapular eruption | No | 2 | Scalp | Yes | 28 |
| 16 | F | 61 | May | No | No | 10 | Scalp | Yes | 13 |
| 17 | F | 29 | May | Fever, myalgia, abdominal pain | No | 3 | Scalp and body | Yes | 28 |
| 18 | F | 27 | June | Fever, thoracalgia, dyspnea | No | 2 | Scalp and body | Yes | 21 |
| 19 | F | 62 | June | Fever, myalgia | No | 0 | Scalp | Still active | - |
| 20 | F | 48 | June | Fever, cough, myalgia | No | 8 | Scalp | Still active | - |
| 21 | F | 41 | July | Fever | Yes (pain) | 3 | Scalp | Still active | - |
| 22 | F | 76 | July | Anosmia, ageusia | No | 11 | Scalp | Yes | 14 |
| 23 | F | 61 | August | Sore throat | Yes (pain, burning sensation) | 2 | Scalp | Still active | - |
| 24 | F | 53 | August | Fever, ageusia | No | 10 | Scalp | Still active | - |
| 25 | F | 72 | August | Fever, sore throat | No | 12 | Scalp | Still active | - |
| 26 | M | 37 | September | Cough | No | 3 | Scalp | Still active | - |
| 27 | F | 48 | October | Fever, anosmia, ageusia, arthralgia | Yes (pain) | 1 | Scalp | Still active | - |
F: female. M: male.
Discussion15
In this case series, we observed 27 patients with acute TE developed after SARS-CoV-2 infection diagnosis. There was a female predominance, as usually occurs in TE. Possible explanations include a higher vulnerability of female hair follicles, easier recognition of hair loss due to longer hairs, and the higher impact of hair loss in women.8,9
In this study, the median latency between the diagnosis of SARS-CoV-2 infection and the development of hair loss was 10 weeks. TE corresponds to a delayed consequence of an abnormal shift in the hair cycle from anagen to telogen, which is responsible for premature shedding of hair, occurring approximately two to three months after a triggering event. Different endogenous and exogenous factors may disrupt the balance of the hair cycle and induce TE, including febrile states.8-11 In our case series, fever was the most frequent symptom of COVID-19, present in 63% of cases. Even so, 15% of patients had asymptomatic SARS-CoV-2 infection. Similar to our observations, hair loss had been a common condition during the 1918 influenza pandemic (also known as Spanish flu), developing 2 to 6 weeks after the onset of the fever, according to historical descriptions.10 Other infectious illnesses have also been associated with TE, such as other viral infections (e.g.: dengue, HIV infection), typhoid, malaria, and tuberculosis.8,13,14
Pathophysiological mechanisms of hair loss in TE and trichodynia are complex and not fully understood.8,10Substance P, a proinflammatory mediator synthesized by neuronal cells and released by cutaneous peripheral nerve terminals, seems to play a major role in trichodynia.15 Inflammation of the small papillary or peripapillary vessels is possibly involved in both TE and trichodynia. Furthermore, some authors suggest that post febrile TE may be secondary to circulating immunocomplexes. Unfortunately, the histopathological examination fails to demonstrate signs of perifollicular inflammation in TE scalp biopsies. This may be due to a deferred biopsy, done when the injurious event is no longer active.10 In our cases, scalp biopsies were not performed. Interestingly, in a third of our cases, increased hair loss developed within the first 3 weeks of SARS-CoV-2 infection. This aspect is comparable to the reports concerning hair loss in dengue,13,16in which hair shedding occurred as early as the first month after acute infection. These descriptions made the authors suggest that the dengue virus may primarily infect human hair follicle dermal papilla cells.16 Regarding COVID-19, multifactorial pathogenesis may explain dermatological conditions associated with SARS-CoV-2 infection.17,18Among them, viral cytopathic effects and inflammatory or immune responses, including stimulation by cytokines and deposition of immunocomplexes,17,18which may also affect hair follicles and induce hair loss.
Generally, TE is a reactive and self-limiting condition. In our case series, TE resolution occurred in the majority of patients and the median duration of the hair loss was 24.5 days. However, the number of days referring to hair loss is an approximate number and should be regarded as a subjective assessment of patients. In fact, if the triggering factor spontaneously resolves or is treated, patients normally experience hair shedding for 2-3 months and a significant cosmetic improvement is expected with full hair restoration after 6-12 months.8
Limitations of this case series include the small size sample and impossibility to estimate the incidence or prevalence of this condition in patients diagnosed with SARS-CoV-2 infection. Furthermore, we cannot exclude an epiphenomenon. The emotional stress involved in the pandemic, even in non-infected people, might also significantly contribute to TE and should not be ignored.19In addition, the confinement recommended by the authorities has led to changes in lifestyle, including eating habits, sleep, outdoor activities, and social life, which may also influence the hair cycle. Further studies are necessary to establish causality between SARS-CoV-2 infection and TE.
Conclusion
Our case series presents the first comprehensive collection of patients with acute TE after SARS-CoV-2 infection. Interestingly, in a third of our cases, TE developed within the first 3 weeks of SARS-CoV-2 infection. Based on these data, we propose that this finding should be considered in COVID-19, especially at the convalescent phase. Despite being a common, reactive, and self-limiting cause of diffuse, nonscarring alopecia, acute TE may manifest as a cataclysmic and alarming phenomenon. Even not progressing to complete baldness, patients who seek medical care are often very concerned about becoming bald.8 Therefore, TE may be a source of significant psychological and emotional distress, which is essential to recognize and reassure patients.8,20
Future studies are necessary to clarify the possible association between SARS-CoV-2 infection and TE, to understand involved pathophysiological mechanisms, and to assess the real incidence of hair loss and trichodynia in patients recently infected with SARS-CoV-2.















