Background
Diabetes mellitus (DM) is a metabolic disease characterised by hyperglycaemia, associated with several organ damage and failure, two major types being described: type 1 diabetes mellitus, a deficit in insulin secretion, and type 2 diabetes mellitus (T2DM), insulin resistance prevailing.1-5 DM represents a major public health matter both in Portugal and globally. (1-2
T2DM prevalence accounts for about 90% of the diabetic population and its prevalence is ever-increasing. (2-3 Its pathogenesis and pathophysiology, in the long term, will lead to a decrease in insulin production, which will become insufficient to meet the body’s needs. (4-5
Obesity, characterized by an increase in body fat, is evaluated in a clinical context by the BMI, is a major chronic disease worldwide with epidemic proportions and affecting all age groups. (3,6-12
According to the Regional Health Profile for the Portuguese Central Region in Portugal (2018 edition) obesity was, in primary health care registrations, the fourth most frequent health problem with a prevalence of 10.2% and DM the fifth with an 8.8% prevalence. There is a prevalence increase as age increases. (13
The Portuguese National Health Service (PNHS) in Central Portugal, is for its administration, comprised of two local health units and six primary health centres clusters differing in their administrative relationship with the board of the Administração Regional de Saúde do Centro (ARS do Centro), a decentralised board of the Portuguese National Health Service, all being super-intended by an administrative board and a Clinical Council, different pharmacological strategies being possible. (13
For the follow-up of T2DM patients in the PNHS, the official e.registrations program has a specific data set in which age, gender, Body Mass Index (BMI), Abdominal Perimeter (AO), values of HbA1c and prescribed medicines, among other issues, are inserted, manually by doctors or nurses.
Different studies have shown that obesity is highly related to insulin resistance in T2DM3,10,14-17 and approximately 80-90% of T2DM patients are overweight (BMI ≥25 kg/m2 and BMI <30 kg/m2)6 or obese (BMI >30 kg/m2). (2-3,6,18 The underlying pathophysiology includes altered adipocyte metabolism, as a consequence of fat overload or as a consequence of pharmacological treatment with drugs such as insulin or sulfonylureas, which favour glucose absorption and abdominal adipose tissue increase. (18-19 There are current therapeutic options, like GLP1analogues and SGLT2i, that can increase glucose excretion, reducing obesity. (18,20-22
For the sake of controlling T2DM and reducing body overweight, alternative to SGLT2i, GLP1analogues operate23-26 in the central nervous system, decreasing appetite, increasing satiety, and leading to weight loss. (25-26 At gastrointestinal tract they delay gastric emptying, and decrease small intestinal peristalsis, thus slowing glucose absorption and lowering the post-prandial glucose peak. (23,25-26
SGLT2i, also called gliflozins, act through an insulin-independent mechanism its primary mechanism being the stoppage of renal reabsorption of glucose in the proximal convoluted tubule - segment S1 and S2 - blocking the sodium-glucose cotransporter SGLT2i. This process results in increased glucose urine excretion lowering blood glucose levels, leading to a negative energy balance, which results in body weight loss. (22,27-28 In addition, they have the potential to delay the development of diabetic nephropathy, (21-22,28-29 which improves the patient’s life quality and is a positive clinical outcome in diabetes treatment.
No data on the correlation between pharmacological treatment with SGLT2i drugs in T2DM patients in the jurisdiction area of the Portuguese National Health Service, the ARS do Centro, were found in the literature.
The present study aimed to compare the progression of obesity, measured by the BMI and Abdominal Perimeter (AP), and the control of T2DM, between 2017 and 2019, according to the pharmacological treatment with SGLT2i versus its absence, in the T2DM population of the ARS do Centro. The quality and extension of the registered data were also studied.
Methods
An observational, retrospective (historical) cohort study was performed in the T2DM population in the Primary Health Care units of the ARS do Centro, a sample being retrieved as representative of such population. All data for this study were anonymously obtained and provided by the informatic services of ARS do Centro. Data from people with the International Classification of Primary Care - 2nd ed. (ICPC2) of non-insulin-treated diabetes from the six primary health care centres clusters was received. T2DM patients from the Local Health Units of Guarda and Castelo Branco, due to their autonomy from the ARS do Centro were not studied. This study design was approved by the Ethics Committee of the ARS do Centro.
Gender, age, time since the diagnosis, AP, BMI, HbA1c in 2017 and 2019, and drugs in the diabetes program sheet of these same people for the years 2017 and 2019 (SGLT2i prescribed versus non-SGLT2i prescribed), were the requested data. Two groups were created according to the treatment or its absence with SGLT2i treatment.
Regarding the descriptive analysis, the qualitative variables were characterised by absolute and relative frequency. Mean and standard deviation were used to characterise age and time since diagnosis. For the remaining variables median and quartiles of the distribution were used, for they did not present a normal distribution globally or in any of the subgroups characterised, regarding medication and gender. The adjustment of the sample distribution to a normal distribution was assessed, case by case, applying the Shapiro-Wilk test and observation of symmetry by the skewness ratio with its standard error, always concluding in asymmetry, justifying the application of non-parametric tests.
The correlation between the variables HbA1C, BMI, and AP in 2017 and 2019, as well as that of the difference between those two assessments - 2017 and 2019 - with age and time since diagnosis using Spearman’s correlation were performed. It was assumed that there was a correlation between the pairs under analysis when the correlation coefficient had values greater than 0.400, in absolute value (|rS| > 0.400), regardless of the p-value associated with the correlation coefficient, given the sensitivity of this statistical test to large sample size.
The Wilcoxon test was used to compare paired samples, both globally and in each group, while the Mann-Whitney test was used to compare the change between both moments regarding gender and medication. The interaction between gender and medication was considered with four levels, and the difference between the two moments regarding that interaction was assessed by the Kruskal-Wallis test.
The analysis was performed in SPSS, v. 27, and was analysed at a 5% significance level.
Results
Sample characterisation
A 127,062 individuals population from ACeS Baixo Mondego, Baixo Vouga, Cova da Beira, Dão Lafões, Pinhal Interior Norte, and Pinhal Litoral was studied. Of this population a sample of 16,012 (12.6%) following the inclusion criteria for medication entered the database: 12,171 were also medicated with SGLT2i (76.0%) and 3,841 were not on iSGLT2 (24.0%) like biguanides, thiazolidinediones, alpha-glucosidase inhibitors, insulin, glinides, and sulphonylureas, no cases with GLP1a in 2017 being found.
Of these 16,012, data distribution revealed more than half of them were from the ACeS Baixo Vouga and Baixo Mondego [5,188 (32.4%) and 3,870 (24.2%) respectively], followed by Dão Lafões (2,613; 16.3%), Pinhal Litoral (2,519; 15.7%), Pinhal Interior Norte (1,413; 8.8%) and Cova da Beira (409; 2.6%), no major discrepancies were found between the sample and the population of the ARS do Centro, equally distributed in terms of gender (male: n=7.971, 48.8%; female: n=8.041, 50.2%), age between 30 and 102 years (mean ± standard deviation: 73.5 ± 10.1 years) and time since diagnosis between 0 and 64 years (mean ± standard deviation: 8.7 ± 4.2 years).
The distribution of HbA1C, BMI, and AP in 2017 and 2019 is different across categories of medication, overall and per gender (p<0.001), except the distribution of BMI in 2017 in male subjects that are the same across different medication categories (p=0.607).
HbA1c
Only 3,600 individuals presented valid HbA1c values both in 2017 and 2019. This sample presents similar characteristics to the overall sample: (M: n=1.787, 49.6%; F: n=1.813, 50.4%), varying in age between 36 and 99 years (mean ± standard deviation: 73.2 ± 10.0 years) and time since diagnosis in the e-registration program of 8.6 ± 4.1 years. Of the 3,600 patients considered, 2,828 (78.6%) were on SGLT2i and the remaining 772 (21.4%) were on one of the other treatments.
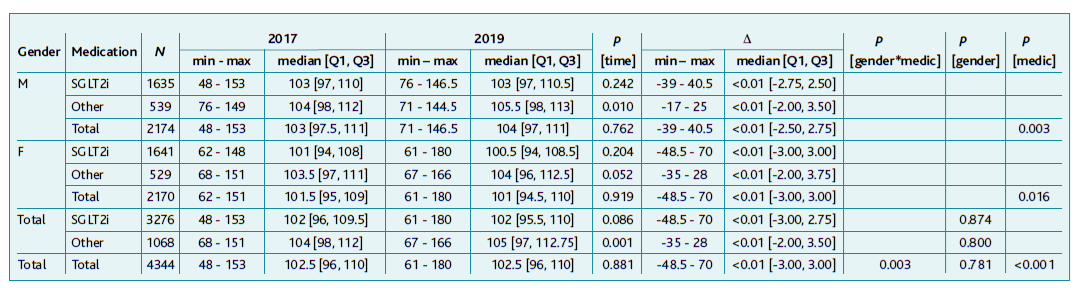
Table 1 shows the difference in HbA1c values between both assessment points, which seems to be independent of age and time since diagnosis, with most of the differences between HbA1C values concentrated between ±2.5%. No correlation was observed between initial and final HbA1C, or percentage difference with age (respectively rS=-0.110, rS=-0.108 and rS=0.007) nor with time since diagnosis (respectively rS=0.057, rS=0.041 and rS=-0.025).
Table 1 Characterisation and comparison of HbA1C values in 2017 and 2019 globally and according to gender and medication
A statistically significant difference was found in median HbA1c values between the two assessment moments, 2017 and 2019, both in the total sample and in the subgroups medicated with SGLT2i in total or per gender (p<0.001), as shown in Table 1. In the subgroups medicated with other drugs rather than SGLT2i, no statistically significant difference was observed, either globally (p=0.983) or per gender(M: p=0.932; F: p=0.932).
As shown in Table 1, there was a higher variance and upward trends with SGLT2i when compared to the group medicated with other drugs (p=0.006), showing a growth dynamic in SGLT2i of 0.014 and in other drugs of 0.003. It was also observed a gender/medication interaction in the variation between the two moments (p=0.044) since there is a trend slightly downward in the group of female subjects medicated with drugs other than SGLT2i, which was not observed in the other three groups where there is an increase in HbA1c between 2017 and 2019. As for total females medicated with SGLT2i, the variation was statistically significant (p=0.049) unlike males (p=0.054), although with a similar effect size with a median magnitude of 0.10 according to Table 1.
BMI
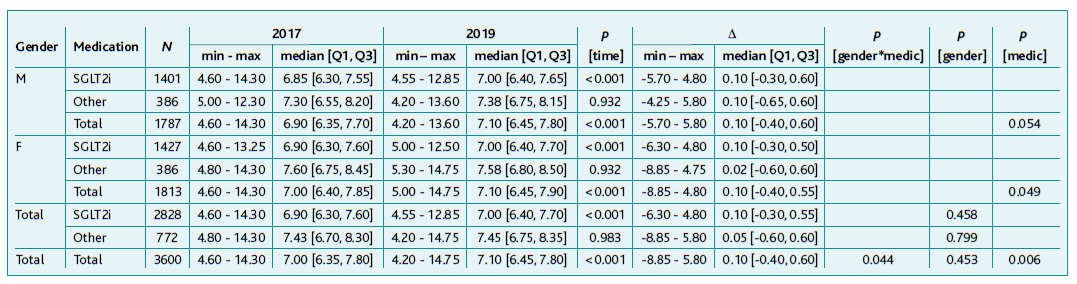
Of the 16,012 only 4,808 individuals had valid BMI values in 2017 and 2019. Like the HbA1c data, the study sample presents characteristics resembling the global sample: (M: n=2.362, 49.1%; F: n=2.446, 50.9%). Age ranged from 33 to 99 years (mean ± standard deviation: 73.2 ± 9.7 years) and time since diagnosis from 0 to 64 years (mean ± standard deviation: 8.8 ± 4.1 years). Of the 4,808 patients considered, 3,741 (77.8%) were on SGLT2i and the remaining 1,067 (22.2%) were using one of the other drugs.
There was no substantial correlation between initial, final or BMI change with age (respectively rS=-0.162, rS=-0.173 and rS=-0.036) nor with time since diagnosis (respectively rS=-0.026, rS=-0.021 and rS=0.006). Highlighting that the difference in BMI values between the two assessment periods seemed to be independent of age and time since diagnosis, with most differences in BMI values concentrated between ± 5kg/m2.
A statistically significant variation in BMI in the group medicated with SGLT2i, both overall (p<0.001) and in each gender (M: p<0.001; F: p=0.007). In the group medicated with other drugs, there was also a statistically significant difference (p=0.004), contrary to what happened by gender with other drugs (M: p=0.177; F: p=0.932), however, males and females overall showed significant variation as well as the total sample (p<0.001).
The reduction was larger in the group treated with SGLT2i (male and female) compared to the group medicated with other drugs, with growth dynamics of -0.015 and -0.005, respectively. It was also observed a gender/medication interaction in the variation between the two moments (p<0.001), because of a greater decrease in BMI, between the two assessments, in women (Table 2).
Table 2 Characterisation and comparison of BMI values in 2017 and 2019 globally and according to gender and medication
Abdominal Perimeter
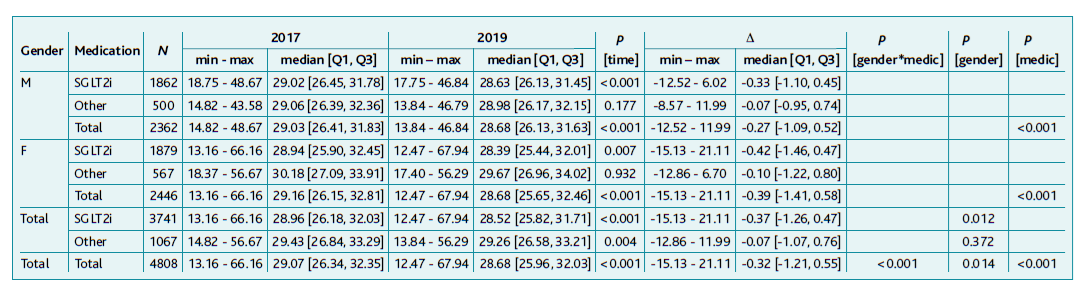
The sample used to assess AP between 2017 and 2019 consisted of 4,344 individuals, having similar characteristics to the overall sample (M: n=2.174, 50.0%; F: n=2.270, 50.0%), with age ranging from 36 to 101 years (mean ± standard deviation: 73.2 ± 10.1 years) and time since diagnosis from 0 to 64 years (mean ± standard deviation: 8.6 ± 3.9 years). Out of the 4,344 patients considered, 3,276 (75.4%) were on SGLT2i and 1,068 (24.6%) on other drugs.
The difference in AP values between the two assessment periods was independent of age and time since diagnosis, with most of the differences between AP values condensed between ± 20 cm. With Spearman’s test, no correlation was found between initial, final, or AP change with age (respectively rS=-0.004, rS=0.003 and rS=0.008) nor with time since diagnosis (respectively rS=-0.039, rS=-0.046 and rS=-0.031).
A statistically significant variation in AP values in the group non-medicated SGLT2i and the SGLT2i medicated one, both overall (p=0.001) and in males (p=0.010) showed an increasing trend in comparison to the remaining, even though the analysis for women was not statistically significant, it showed a value of p=0.052. It was observed an increase of 1.5cm in median values of AP in males (growth dynamic of +0.010) and only 0.5 cm in females (growth dynamic of +0.005).
Regarding growth dynamics, SGLT2i medicated ones showed a null value while other drugs presented a 0.007 growth.
No statistically significant variation was found in any of the analyses regarding SGLT2i.
An interaction between gender/medication in the variation between 2017 and 2019 (p=0.003) was perceived, with both genders presenting growing trends in AP, greater in male subjects (p=0.003) than in female ones (p=0.016). A statistically significant variation in the group medicated with other drugs when compared with patients under SGLT2i was found (<0.001).
Discussion
This study examined a sample of the population with T2DM in Portugal’s central region. According to the inclusion criteria, from the 127,062 individuals in the study, only 16,012 were registered as being under pharmacological treatment. This short number deserves interventions either to the doctors to tick the right button or to the informatics responsible for automatic filling-in when the anti-diabetic class is prescribed. Of the 16,012 studied individuals who were medicated with SGLT2i or other medication, only 3,600 presented valid HbA1C, BMI, and AP values in 2017 and 2019. Therefore, we may be facing health professionals not registering the values in clinical appointments (a task to be performed both by nurses and doctors) or patients not regularly attending scheduled diabetes consultations. Thus, the problem of lack of recorded data is a dilemma to debate and presents a limitation to this study or others based on this type of data.
Obesity was expected to be observed in the majority of T2DM patients. Simultaneously, an increase in obesity prevalence or worsening was expected in patients on therapies that did not include SGLT2i a favourable outcome being expected if SGLT2i was present. The reduction of obesity by BMI and AP was expected to be associated with a decrease in HbA1c value regardless of the therapy.
The target control value for HbA1c may vary from patient to patient due to concomitant literacy, other simultaneous morbidities, and patient’s age (patients with 65 years or older target value <7.0% and under 65 years target value <6.5%30).2,18 The present study showed no correlation between initial and final HbA1C values, nor difference with age or with time since diagnosis since the |rS| was always below 0.110. This was also observed for BMI, maximum |rS|=0.173, and PA, maximum |rS|=0.046. According to data from efficacy studies, these results were not anticipated. It was expected that younger and earlier diabetes diagnoses would present poorly controlled DM leading to higher HbA1c and BMI values as well as central obesity with greater AP values. (30-32
Although SGLT2i has a larger positive variation than the other drugs, this study only covered a two-year observational period, so, even if there was a greater increase in HbA1c in patients under this therapy (with a growth dynamic of +0.014) when compared with other drugs (growth dynamic of +0.003, one must consider the results in the long term and the effects of the decrease of BMI and AP in HbA1c that may not be prompt, but eventually influence in each other’s growth dynamics. It is to be noticed that this growth dynamics influences values that, in the case of those with SGLT2i, were statistically lower in 2017.
In the SGLT2i group, the difference was significantly different, the effect observed in the median difference was never greater than 0.10%, but the variation between these two points in time was from moderately controlled median values to borderline values (6.90% to 7.00%). However, the non-SGLT2i patients, even with a lesser variation, always presented values in the uncontrolled range (from 7.43% to 7.45%). Thus, it is important to continue to investigate these values, pursuing this cohort study.
Regarding BMI, the presence of overweight or obesity is obvious in the sample, with more than 75% of records with values ≥ 25kg/m2 in both years, as anticipated. (3,6,9-10,14,18 As expected, and as discussed in other studies, the reduction variation in the BMI was statistically significant in individuals medicated with SGLT2i and also with other drugs globally, and for this group these results deserve future causative studies. (32 Even though, the effect was greater with SGLT2i22,27-28 than in non-SGLT2i medicated, the gap in median values in both being <1 kg/m2 (SGLT2i 0.37 kg/m2 and 0.07 kg/m2 in non-SGLT2i medicated. However, one would expect a more obvious difference between the different treatments. The slight reduction observed with non-SGLT2i medicated was not anticipated since we expected an increase in obesity prevalence or worsening in patients on therapies that do not include SGLT2i. This may be due to proper patient care to keep the disease under control. The interaction gender*medication observed between the two moments showed that women had a greater reduction in BMI with both therapies, deserving further investigations. The distribution of BMI values in 2017 in male subjects is the same across different medication categories (p=0.607). As a bias, we do not know the effect of the knowledge by patients of a new treatment, which has probably been biased to have thinning properties.
Concerning AP, a greater positive variation was observed in the group medicated with other drugs, showing a more pronounced upward trend globally and in males, which was not observed in the SGLT2i treatment group. As for the gender*medication interaction, an increasing trend in males and females in the non-SGLT2i medicated was observed, once more something to be studied in the future. This was anticipated since other drugs were not weight loss promoters, however, a decrease in AP would be expected in SGLT2i users accompanying the decrease in BMI18-19,22,27-28 as already, instead of a plateau of this parameter.
One must acknowledge that T2DM patients must be considered and treated holistically. Therefore, attention should be attracted to other variables that may alter treatment results, individualised treatment prescription, and therapeutic inertia besides beliefs about medicines and diabetes. (34-35
The aforementioned problems require a multilevel resolution, starting with doctor-patient relationship improvement, inclusion of patients in therapeutic decisions, and monitoring the side effects of therapies to achieve better adherence to treatment, which must mandatorily include a healthy lifestyle (diet and exercise) to enhance the effects of anti-diabetic drugs. (36 This implies better patient enablement and better control of chronic diseases for a better quality of life. It is of paramount importance to invest in more accurate medical records, patient and doctor centred instead of management centred.
The main strength of this study is a very large sample of T2DM patients from Central Portugal, with specific inclusion criteria, which allows us to understand the treatment effects in a real clinical context and its effectiveness. As for the limitations, we should mention the fact that this is a retrospective study only seeking to know about differences occurring from medicines prescription. Therefore, adherence and maintenance in therapeutics, the impact of socioeconomics in the control of T2DM, and beliefs about medicines were not studied. There may be a criticism about the validity of the studied data, made by doctors and nurses in an e.registration support program (S-Clínico). Still, if we are not to trust clinical records, then what should we trust? Nevertheless, a worrisome problem comes from the lack of existing data from many T2DM patients. So many computer resources exist, why are they not being exploited at their best?
These results must also be carefully read for, data layouts were difficult to obtain namely for the knowledge of what medications the patient was in.
The present results are not similar to efficacy studies (clinical assays), but the time length is also different, these being shorter. Therefore, more follow-up studies must be made, probably in other geographic contexts and even in randomised studies.
Conclusion
SGLT2i attained a greater and significant decrease in BMI when compared with the effect of non-SGLT2i medication. Still, the median variation was lower than 1 kg/m2.
AP in SGLT2i users remained quite stable, in contrast to non-SGLT2i medicated patients, whose AP value increased. However, an increase in obesity prevalence or worsening in patients on therapies that do not include SGLT2i was not observed.
In this two-year observational study, the effectiveness of SGLT2i to decrease HbA1c values when compared with non-SGLT2i medicated did not reach clinical superiority. However, we should not take this value as the endpoint and think about the future of what these effects will represent in the long term of T2DM follow-up.
Real-world data must continue to be studied prospectively to draw fuller conclusions.
Acknowledgements
Special acknowledgment to Conceição Saraiva, from the informatics services of ARS do Centro, for the time spent collecting data, which enabled this work to be carried out.
Authors contribution
Conceptualization, MP, LMS, and BO; methodology, MP, LMS, and BO; software, BO; validation, MP, and BO; formal analysis, BO; investigation, MP, and LMS; resources, LMS; data curation, MP, LMS, and BO; writing - original draft preparation, MP, LMS, and BO; writing - review and editing, MP, LMS, and BO; visualization, MP, LMS, and BO; supervision, LMS, and MO; project administration, MP, LMS, and BO. All authors have read and agreed to the published version of the manuscript.