Case Report
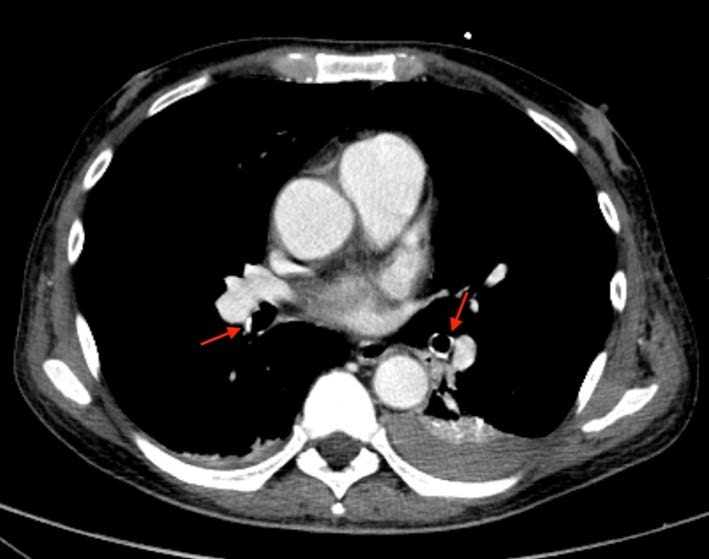
A 57-year-old man with long-lasting chronic kidney disease due to post-streptococcal glomerulonephritis was referred to our nephrology clinic to perform a second renal transplantation. The patient had a 28-year history of hemodialysis treatment and a first kidney transplant failure. Impaired renal function with normal calcemia, slightly elevated phosphorus, and hyperparathyroidism were observed through laboratory tests. During the previous 3 months, he had a history of non-productive cough with persisting dyspnea. His physical examination was unremarkable. The chest radiograph (Fig.1) revealed numerous small nodular opacities with patchy areas of parenchymal opacification. The nodules were predominant in the upper lung zone and calcification of the nodules was evident. Computed tomography (CT) of the chest showed multiple ground-glass opacities associated with poorly defined centrilobular nodules with ground-glass attenuation and coarse areas of calcification (Fig. 2). There was a symmetrical distribution involving both upper lung lobes and also bronchial calcifications (Fig. 3). There were no visible signs of pleural effusion, pulmonary fibrosis, or lymphadenopathy. Based on these findings and in this setting of end-stage renal disease, the diagnosis of metastatic pulmonary calcification (MPC) was made. Attempt to normalize the patient’s calcium and phosphate biochemistry has been the mainstay of therapy. Following treatment, the patient’s imaging abnormalities have been stable, and he is now waiting for a new transplant match.
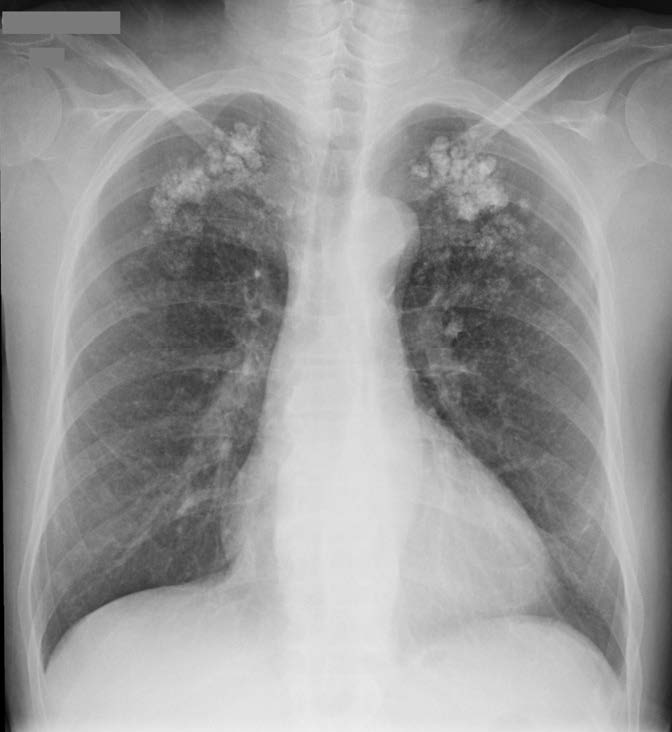
Figure 1: Chest radiograph showing numerous small nodular opacities with patchy areas of parenchymal opacification in both upper lung zone.
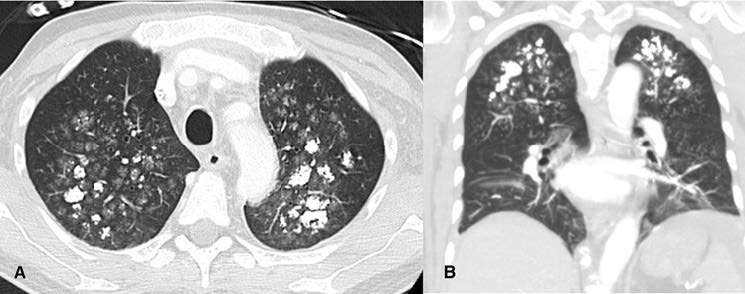
Figure 2: (A) Axial and (B) Coronal chest CT scan showing multiple ground-glass opacities associated with poorly defined centrilobular nodules with coarse areas of calcification, with a symmetrical distribution involving both upper lung lobes.
Discussion
MPC is a metabolic lung disease characterized by diffuse interstitial deposition of calcium in the normal lung parenchyma.1,2This disorder usually occurs due to conditions that induce hypercalcemia, which can be both benign and malignant. Among benign etiologies, end-stage renal disease with secondary hyperparathyroidism, primary hyperparathyroidism, hypervitaminosis D and osteoporosis are the commonest.3 Renal or liver transplantation and cardiac surgery4,5can also be included in this category. In the group of malignant etiologies, multiple myeloma is the most frequent cause of cancer-related hypercalcemia, being membrane and alveolar capillary walls, with no substantial desmoplastic reaction.11 Although, in several cases, a desmoplastic reaction may develop with consequent interstitial fibrosis appearance. This fibrotic condition is thought to be the main cause for respiratory symptoms development and onset of lung function disorders.11,12,13 Clinical manifestations of MPC are usually minimal, but occasionally dyspnea and chronic non-productive cough may be present. When pulmonary calcification is asymptomatic, with slow clinical deterioration, the condition is rarely diagnosed.3 Patients with significant calcifications may be completely asymptomatic, showing no direct correlation between the extent of macroscopic calcifications and clinical symptomatology.14
Chest radiographic findings are variable and non-specific for MPC diagnosis. Due to the low sensibility to depict small amount of calcification, chest radiograph is usually normal. A pattern of confluent and patchy airspace opacities simulating infection or pulmonary edema may responsible for 20-30% of the cases.6 Other malignant causes that can lead to this condition are leukemia, parathyroid carcinoma, lymphoma, breast cancer and malignant melanoma.7,8,9 In our case, there were several factors predisposing to MPC including hypercalcemia, hyperparathyroidism, and chronic kidney failure with a history of hemodialysis.
Due to the high levels of serum calcium and phosphate, calcium deposits can occur in any organ of the body, with a predilection to sites disposed to alkalinity, such as the lungs, kidney, and stomach. In the lungs, calcium salt deposits are usually located in the alveolar epithelial basement membranes, with an affinity for elastic tissue. It can also appear in the walls of the bronchioles and pulmonary vessels, explaining the interstitial and centrilobular location of the findings. Sporadically, calcifications can also be found in the large airways.3,10 In moderate situations, there is calcium deposits in the alveolar epithelial basement be found.11 Also, a diffuse interstitial process or confluent calcified nodules are other possible forms of presentation. Imaging with CT is a sensitive diagnostic tool in pulmonary calcification diagnosis and much more accurate on its detection. Three different patterns of MPC have been described in literature: - multiple millimetric diffuse calcified nodules, which can be distributed through the whole lung or have a predilection for the apices; - diffuse or patchy areas of ground-glass opacity/consolidation; - confluent high-attenuation parenchymal consolidation with a lobar distribution, mimicking lobar pneumonia.9 The most common parenchymal finding on chest CT is numerous ill- defined fluffy centrilobular nodules that may contain foci of calcification. Well-defined areas of dense consolidation can be seen in cases of severe interstitial calcification. These findings are the consequence of calcium deposition in the alveolar walls around the terminal bronchioles. In addition to parenchymal pulmonary abnormalities, calcification of the chest wall vessels, bronchial walls and myocardium are also a possible findings.13 In our case, CT scan showed some typical MPC findings such as multiple bilateral centrilobular ground-glass opacities and areas of consolidation distributed throughout the upper lung zones, and bronchial calcifications. The higher ventilation-perfusion ratio in the upper lobes leads to a lower end capillary PCO2 and a consequent higher pH. This physiological phenomenon is the main reason why calcium deposits are mainly located in the upper lung area instead of the lung bases.15 Innovating imaging techniques such as spectral-detector computed tomography (SDCT) may be highly useful to clarify and confirm the suspicion of calcified nodules or ground-glass opacities present in conditions as MPC. A recently published case showed that when using a calcium-suppressed images in SDCT, the ground-glass opacities and parenchymal calcifications “vanish” in opposite to other pulmonary infiltrations such as pneumonia and pulmonary edema, which would be unchanged by calcium suppression.16 This is possible due to the additional spectral information used in this technique which allows the subtraction of calcium from the reconstructed images, once materials have attenuation profiles at different energy levels.17
Bone scintigraphy using technetium-99m-MDP is a sensitive and specific technique for an early detection of MPC.18 Lung parenchyma with abnormalities by MPC show increased uptake of the radioactive isotope, even in the absence of radiographical abnormalities. Accordingly to some authors, the use of scintigraphy should be part of evaluation of patients with dyspnea and chronic renal failure.3
MPC is the commonest cause for multifocal pulmonary parenchymal calcifications in patients diagnosed with chronic renal disease. The typical upper lung predilection for the calcification in association with calcification of the vessels of the chest wall are important directions for the diagnosis.19 Depending on the pathophysiological mechanism, lung calcium deposition can occur into two different ways. In MPC, as described before, the serum mineral iron imbalance leads to calcium salt deposition into normal lung tissue. On the other hand, dystrophic calcification differs from the first one since the calcium and phosphate serum levels in these cases are normal.1 This last pattern is usually found in the context of a previously damage lung parenchyma due to infections as tuberculosis, histoplasmosis, or chickenpox. Also, some metastatic malignancies (osteogenic sarcoma, chondrosarcoma, mucin-producing adenocarcinomas, and thyroid malignancies), chronic hemorrhagic conditions, depositions diseases, and idiopathic disorders such as pulmonary alveolar microlithiasis may be included in the list of differential diagnosis for calcified lung nodules. Alveolar microlithiasis has small calcified micronodules, typically located in the lower zones and paracardiac regions. Miliary tuberculosis infection is characterized by millimetric well- defined nodules, distributed throughout both lungs, that may calcify. In silicosis, the nodules are typically located in the middle and upper long zones and are often associated with hilar and mediastinal lymph nodes with peripheral egg-shell calcification.9,19 In situations where MPC present as a diffuse, mainly interstitial, and with some air-space opacities, a misdiagnosis of pneumonia or pulmonary edema may be made.20
Calcium and phosphate biochemistry normalization is defined as the gold standard treatment for MPC itself since the therapy for this condition is based on the underlying causes. While the majority of asymptomatic patients with chronic renal failure and non-progressive MPC do not require intervention, treatment including parathyroidectomy or bisphosphonates have been recommended for symptomatic patients, to normalize the calcium levels and prevent the progression of calcification.21 The resolution of pulmonary calcification in patients with chronic renal failure can occur after correction of hypercalcemia due to renal transplantation, parathyroidectomy or dialysis.9
In conclusion, MPC is a frequently asymptomatic and misdiagnosed complication associated with end-stage renal disease. Patients with chronic renal disease and undergoing dialysis have a proclivity to develop MPC, particularly if these patients present with pulmonary symptoms or unexplained radiographic changes. Since it may progress to irreversible lung damage and subsequently respiratory failure, the knowledge of its imaging findings is crucial for a correct diagnosis and management of patients. CT scan and Tc99m-MDP bone scanning can be very helpful for the detection of MPC, and the implementation of techniques as dual-energy-based calcium-specific images may improve the patient’s diagnosis and follow-up in the future.